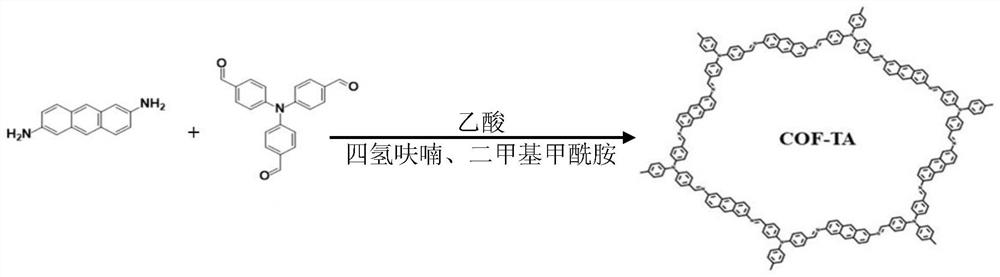
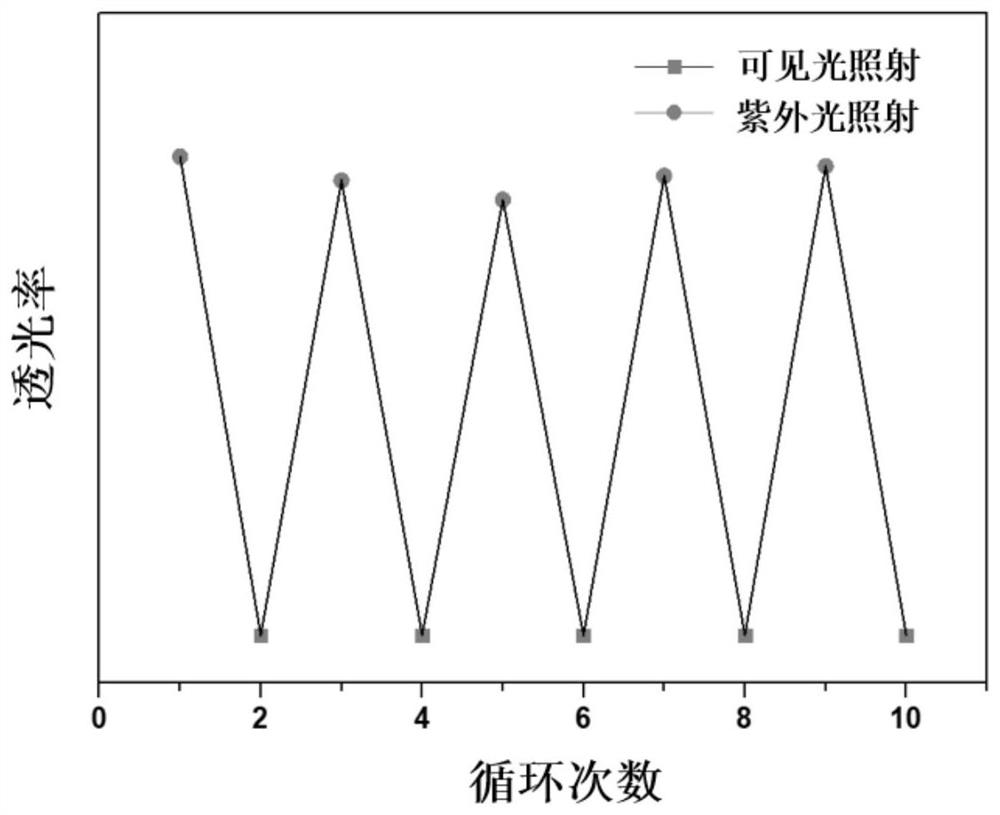
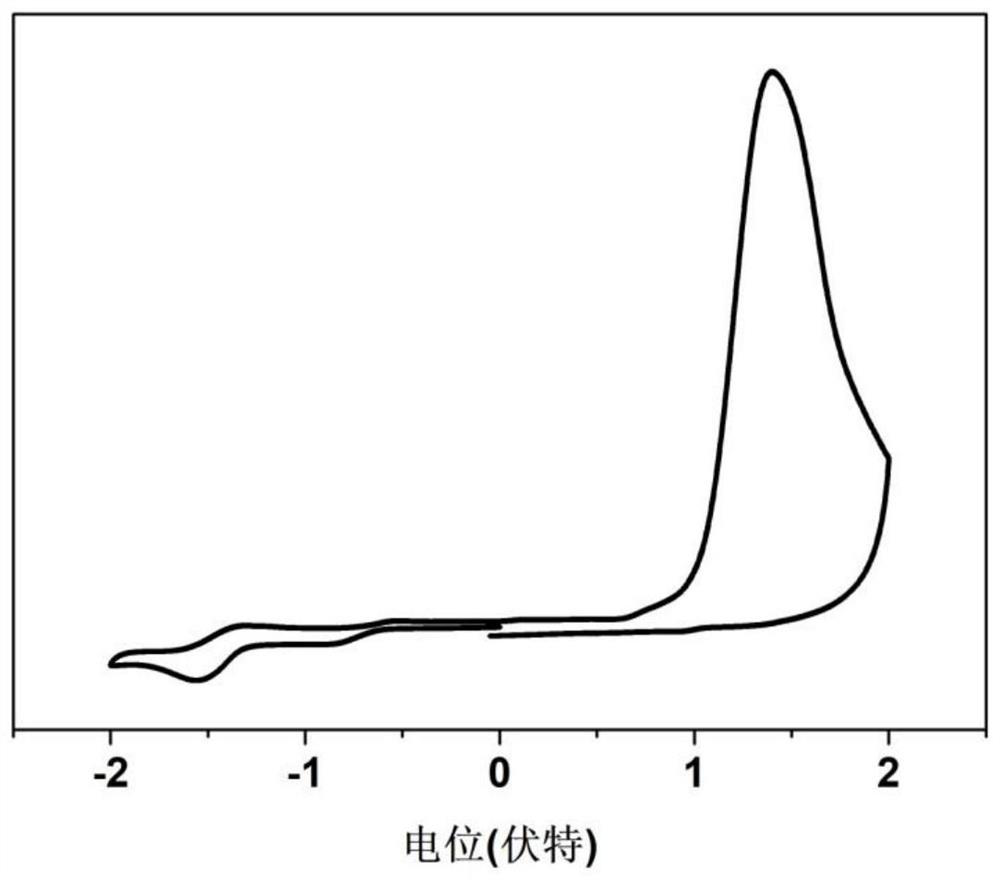
Conjugated organic framework material COF-TA based on anthracene unit and preparation method thereof
A COF-TA, organic framework technology, applied in the field of conjugated organic framework material COF-TA and its preparation, can solve problems such as increasing the degree of reaction, achieve high spatial symmetry and conjugation, good electrical properties and light absorption performance effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0027] refer to figure 1 , first place 2,6-anthracenediamine, tris(4-formylphenyl)amine, solvent, and catalyst in a sealed tube, and in every 3ml of solvent, 2,6-anthracenediamine, tris(4-formylphenyl) The addition amount of phenyl)amine and catalyst are respectively: 0.08mmol, 0.2mmol, 0.4mmol, and then the deoxygenation operation is performed on the sealed tube, and then the polycondensation reaction is carried out at 120°C for 3 days under the protection of nitrogen, and finally the product is placed in Wash and purify with eluent in a Soxhlet extractor, and dry to obtain the product. Among them, the catalyst is acetic acid; the deoxygenation operation is specifically: first place the sealed tube in liquid nitrogen to freeze, then vacuumize, and finally thaw, the deoxygenation operation is repeated three times and all are carried out in a nitrogen atmosphere; the eluent is petroleum ether, The mixture of acetone and methanol with a volume ratio of 1:1:1, and the solvent is...
Embodiment 2
[0029] First place 2,6-anthracenediamine, tris(4-formylphenyl)amine, solvent, and catalyst in a sealed tube, and in every 2ml of solvent, 2,6-anthracenediamine, tris(4-formylphenyl) The addition amount of amine and catalyst are respectively: 0.1mmol, 0.15mmol, 0.5mmol respectively, then deoxygenation operation is performed on the sealed tube, and then the polycondensation reaction is carried out at 135°C for 7 days under the protection of argon, and finally the product is placed in Wash and purify with eluent in a Soxhlet extractor, and dry to obtain the product. Among them, the catalyst is p-toluenesulfonic acid; the deoxygenation operation is as follows: first place the sealed tube in liquid nitrogen to freeze, then vacuumize, and finally thaw, the deoxygenation operation is repeated three times and all are carried out in a nitrogen atmosphere; The agent is a mixture of dichloromethane and chloroform at a volume ratio of 1:2, and the solvent is tetrahydrofuran.
Embodiment 3
[0031] Put 2,6-anthracenediamine, tris(4-formylphenyl)amine, solvent, and catalyst in the sealed tube firstly, and in every 4ml of solvent, 2,6-anthracenediamine, tris(4-formylphenyl) The addition amount of amine and catalyst are respectively: 0.05mmol, 0.25mmol, 0.3mmol respectively, then deoxygenation operation is performed on the sealed tube, and then the polycondensation reaction is carried out at 150°C for 1 day under the protection of argon, and finally the product is placed in Wash and purify with eluent in a Soxhlet extractor, and dry to obtain the product. Among them, the catalyst is formic acid; the deoxygenation operation is as follows: first place the sealed tube in liquid nitrogen to freeze, then vacuumize, and finally thaw, the deoxygenation operation is repeated three times and all are carried out in a nitrogen atmosphere; the eluent is ethyl acetate , the solvent is petroleum ether.
[0032] Examples 1-3 can all obtain the conjugated organic framework material...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com