8-hydroxyquinoline derivative iridium (III) complex as well as preparation method and application thereof
A technology of hydroxyquinoline and derivatives, applied in the field of medicine, to achieve high anti-tumor activity and good target inhibition effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
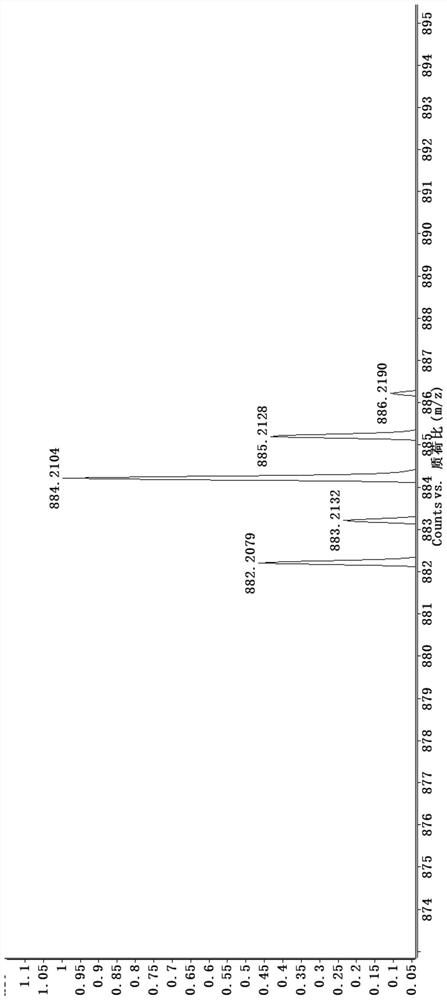
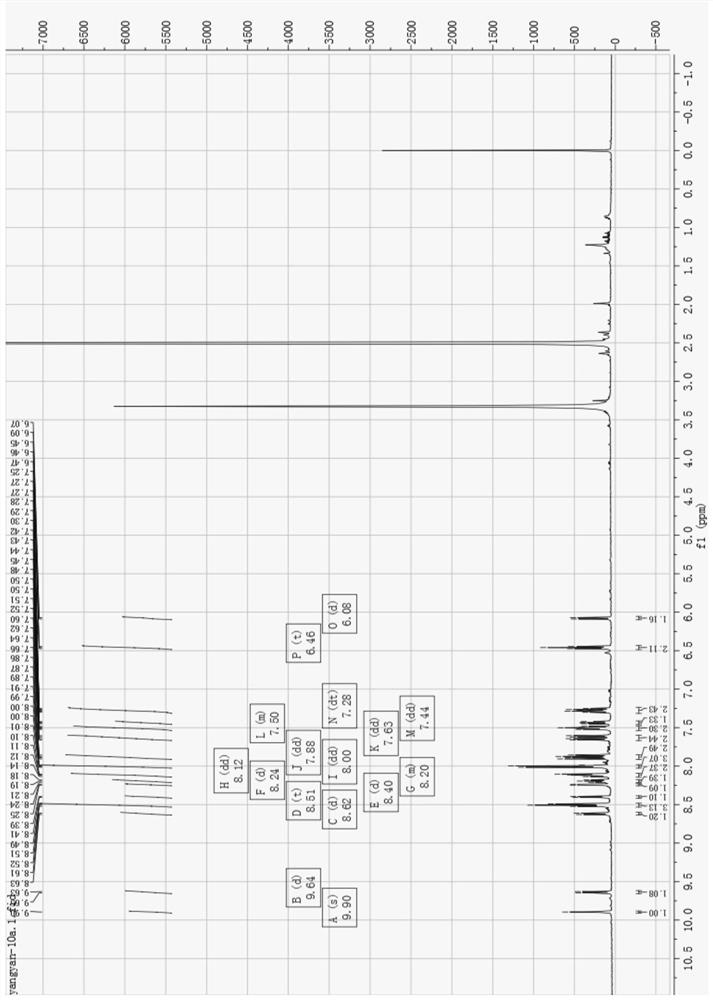
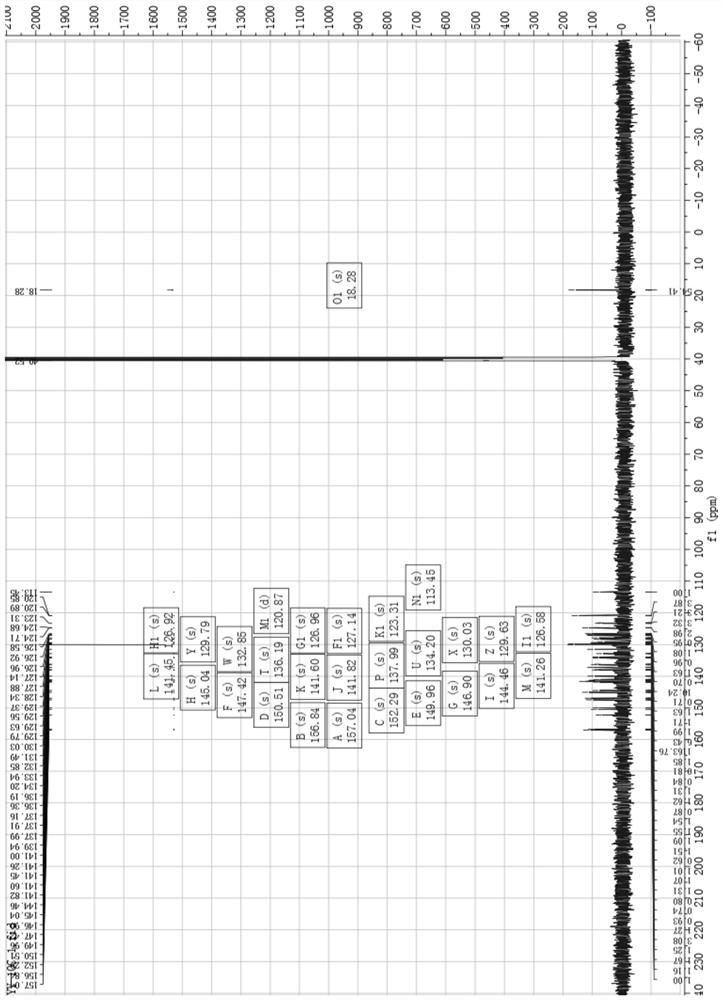
[0039] (1) 8-hydroxyquinoline derivative iridium (Ⅲ) complex [Ir (3a) (BQ) 2 ] preparation and characterization:
[0040] Such as Image 6 As shown, weigh compound 4 and compound 3a (8-hydroxyquinoline derivative) according to the molar ratio of 1:1.5, put them into the container, then add ethylene glycol, heat and reflux for 13h under the protection of argon, and obtain a dark red clear solution ; Cool to room temperature, add water to dilute, and then add saturated ammonium hexafluorophosphate solution, which produces a large amount of red precipitate; filter with suction, wash with water and ether, and dry; dissolve the dried crude product with acetonitrile, and filter with neutral alumina Column separation; use V (dichloromethane): V (acetonitrile) = 3: 1 mixed solvent to collect the yellow component, then distill under reduced pressure and spin dry to remove the solvent to obtain a khaki solid product, which is 8-hydroxyquinoline Phyloline derivatives iridium (Ⅲ) comple...
Embodiment 2
[0052] 8-Hydroxyquinoline derivatives iridium (Ⅲ) complexes [Ir (3a) (BQ) 2 ] The synthetic route such as Image 6 Shown:
[0053] (1) Preparation of compound 1: add 600mL of concentrated hydrochloric acid (pre-placed in the refrigerator to freeze, the concentration of concentrated hydrochloric acid is at 37% volume fraction) and 14.5g of 8-hydroxyquinoline in a 1L round bottom flask, and heat the temperature to 40°C , after the 8-hydroxyquinoline is completely dissolved, add 53g NaClO in batches 3 (The addition was completed within 60 minutes), and after the addition was completed, the stirring was continued at 40° C. for 2 h. After the reaction was completed, dilute to 2L with ice water and dilute with CH 2 Cl 2 (6 × 250mL) extraction, combined organic phase, washed with 3 × 200mL of distilled water, vacuum rotary evaporation of the solvent to obtain a yellow solid, filtered the precipitate, and the solid was recrystallized three times with 40mL of methanol to obtain 6,7...
Embodiment 3
[0059] 8-Hydroxyquinoline derivatives iridium (Ⅲ) complexes [Ir (3a) (BQ) 2 ] The synthetic route such as Image 6 Shown:
[0060] (1) Preparation of compound 1: carefully add 725mL of concentrated hydrochloric acid (placed in the refrigerator to freeze in advance, the concentration of concentrated hydrochloric acid is at 35% volume fraction) and 14.5g of 8-hydroxyquinoline in a 1L round bottom flask, and heat to 45 ℃, after the 8-hydroxyquinoline is completely dissolved, add 64g NaClO in batches 3 (The addition was completed within 60 minutes), and after the addition was completed, the stirring was continued at 45° C. for 1 h. After the reaction was completed, dilute to 2L with ice water and dilute with CH 2 Cl 2 (6 × 250mL) extraction, combined organic phase, washed with 3 × 200mL of distilled water, vacuum rotary evaporation of the solvent to obtain a yellow solid, filtered the precipitate, and the solid was recrystallized three times with 40mL of methanol to obtain 6,7...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com