Method for preparing cyclopentanone
A technology of cyclopentanone and epoxycyclopentane, applied in the field of organic chemical industry, can solve the problems of equipment corrosive environment, complex source of raw materials, low yield, etc., and achieve the effects of environmental safety and friendliness, abundant source of raw materials and high yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
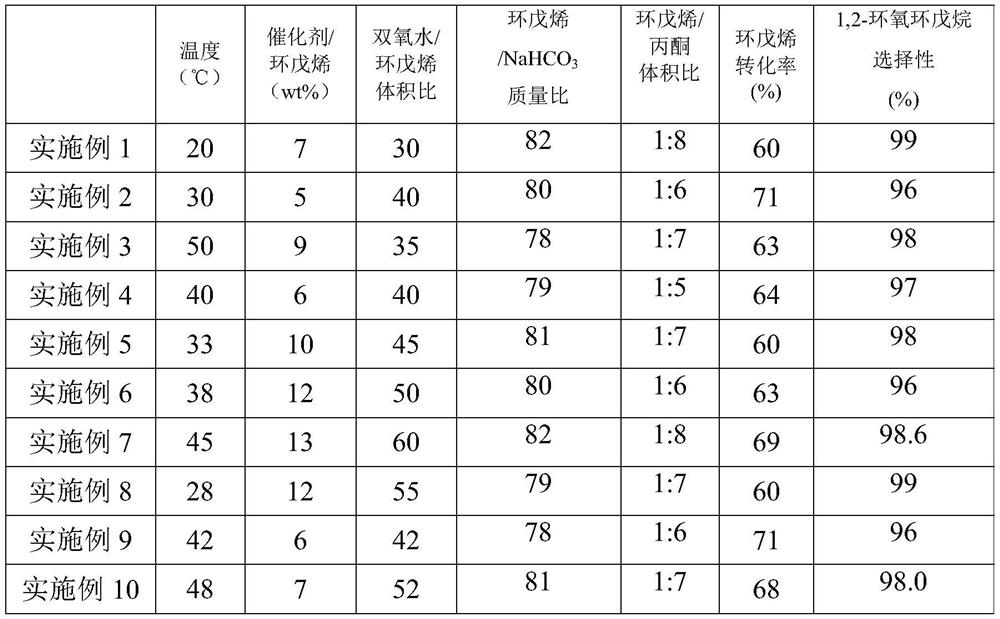
Embodiment 1~10
[0038] 1. Oxidation reaction
[0039]The conversion of cyclopentene and the selectivity of 1,2-epoxycyclopentane are calculated according to the following formula:
[0040]
[0041]
[0042] Among them, (cyclopentene content) in Indicates cyclopentene import molar content; (cyclopentene content) out Indicates the molar content of cyclopentene outlet; (1,2-epoxycyclopentane molar content) indicates the molar content of 1,2-epoxycyclopentane in the reaction liquid after oxidation reaction; (cyclopentene molar content) in represents Cyclopentene import molar content; (cyclopentene molar content) out means cyclopentene export molar content.
[0043] The reactor for the oxidation reaction is a tank reactor, and cyclopentene and acetone are added to the reactor in proportion, and then TS-1 molecular sieve catalyst is put in in proportion. The catalyst is provided by Sinopec Shanghai Petrochemical Research Institute, and the additive NaHCO 3 and hydrogen peroxide for an oxid...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com