The preparation method of 2-fluoroethylamine hydrochloride
A technology of fluoroethylamine hydrochloride and fluoroethyl phthalimide, which is applied in the field of preparation of 2-fluoroethylamine hydrochloride, can solve the problem of unfavorable industrialized production, requiring multiple recrystallizations, and being time-consuming Cumbersome and other problems, to achieve the effect of solving industrial production problems, low cost and low waste liquid volume
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
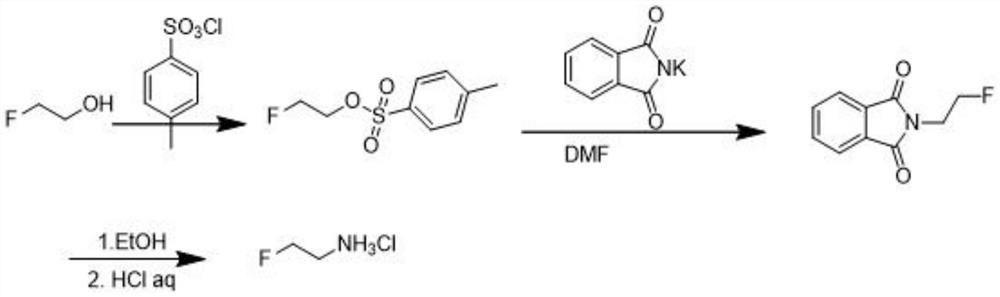
[0022] The preparation method of 2-fluoroethylamine hydrochloride proposed by the present invention, the method steps are as follows:
[0023] (1) Synthesis of 2-fluoroethane phthalimide: add 254g (2mol) of 2-fluoro-1-bromoethane and 380g (2.05mol) of phthalate in a 2L reaction vessel Potassium imide, dissolved with 1.5L of N,N-dimethylformamide (DMF) under mechanical stirring, heated to 100°C for 8h reaction, distilled under reduced pressure, the amount of DMF recovered was 1.3L, and 1.3L of water was added After stirring, a large amount of white solids were precipitated, filtered, and the filter cake was rinsed twice with 300 mL of water, and dried in vacuo to obtain 340 g (1.76 mol) of the intermediate 2-fluoroethane phthalimide with a yield of 88%.
[0024] (2) Synthesis of 2-fluoroethylamine hydrochloride: 340 g (1.76 mol) of the 2-fluoroethyl phthalimide intermediate prepared in S1 was added to a 3 L reaction vessel and stirred with 2L of ethanol was dissolved, then 121...
Embodiment 2
[0026] (1) Synthesis of 2-fluoroethane phthalimide: 254 g (2 mol) of 2-fluoro-1-bromoethane and 296.5 g (1.6 mol) of phthalic acid were added to a 2L reaction vessel Potassium carboximide, dissolved with 1.5L of N,N-dimethylformamide (DMF) under mechanical stirring, heated to 100°C for 8h reaction, distilled under reduced pressure, the amount of DMF recovered was 1.3L, 1.3L was added After stirring with water, a large amount of white solids were precipitated, filtered, and the filter cake was rinsed twice with 300 mL of water, and dried under vacuum to obtain 289 g (1.495 mol) of intermediate 2-fluoroethane phthalimide with a yield of 75%.
[0027] (2) Synthesis of 2-fluoroethylamine hydrochloride: 289 g (1.495 mol) of the 2-fluoroethane phthalimide intermediate prepared in S1 was added to a 3L reaction vessel and stirred with a mechanical 1.7L of ethanol was dissolved, then 74.8g (1.2mol) of 80% hydrazine hydrate was added dropwise, the temperature was raised to 50°C for reac...
Embodiment 3
[0029] (1) Synthesis of 2-fluoroethane phthalimide: 254g (2mol) of 2-fluoro-1-bromoethane and 407.4g (2.2mol) of phthalate were added to a 2L reaction vessel Potassium carboximide, dissolved with 1.7L of N,N-dimethylformamide (DMF) under mechanical stirring, heated to 100°C for 8h reaction, distilled under reduced pressure, the amount of DMF recovered was 1.5L, 1.5L was added Water was stirred to precipitate a large amount of white solids, filtered, and the filter cake was rinsed twice with 300 mL of water, and dried in vacuo to obtain 347.7 g (1.8 mol) of 2-fluoroethane phthalimide intermediate, with a yield of 90%. .
[0030] (2) Synthesis of 2-fluoroethylamine hydrochloride: 347.7 g (1.8 mol) of the 2-fluoroethane phthalimide intermediate prepared in S1 was added to a 3L reaction vessel and stirred under mechanical stirring. Dissolve with 2.2L ethanol, then add dropwise 123.7g (1.98mol) of 80% hydrazine hydrate, heat up to 50°C for reaction 1h, then heat up to reflux for 8...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com