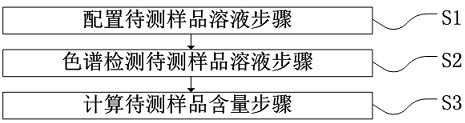
Liquid chromatographic analysis method for detecting content of rearranged ester
A technology of liquid chromatography analysis and liquid chromatography, which is applied in the field of liquid chromatography analysis to detect the content of rearranged esters, can solve the problems of complex response factor gap of product impurities, unclear chromatographic conditions, etc., and achieve short analysis time, heavy Good performance and good peaking effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0059] 1.1 Reagents and instruments
[0060] Methanol: HPLC;
[0061] Rearranged ester standard sample: self-made purity 98.9%;
[0062] Analytical balance: 0.0001g;
[0063] Organic phase microporous membrane: 0.22μm;
[0064] Liquid chromatograph: U3000, Thermo Fisher, equipped with UV detector, equipped with
[0065] Autosampler;
[0066] Capillary column: C18 liquid chromatography column, 150mm×4.6mm×2.6μm, Thermo
[0067] flying company;
[0068] 1.2 Chromatographic method
[0069] a) Mobile phase: A: acetonitrile or methanol; B: 1% phosphoric acid or acetic acid aqueous solution
[0070] b) mobile phase ratio A:B=80:20;
[0071] c) Injection volume: 20.0 μL;
[0072] d) Column thermostat: 30°C;
[0073] e) Flow rate: 1mL / min;
[0074] f) Detection type: UV detector;
[0075] g) Detection wavelength: 222nm;
[0076] h) Chromatographic column: C18 liquid chromatography column, 150mm×4.6mm×2.6μm;
[0077] 1.3 Draw standard curve
[0078] Take the standard wor...
Embodiment 2
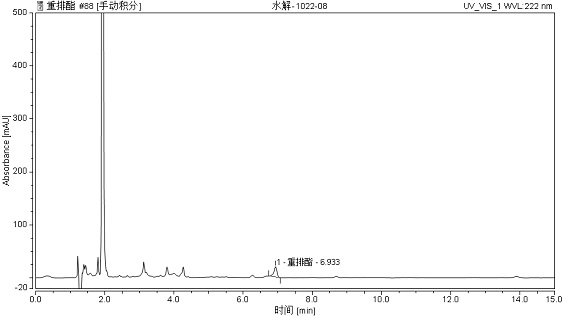
[0093] Embodiment 2 includes most of the technical features of Embodiment 1, and the difference with Embodiment 1 is that in Embodiment 2, the sampling position is the raw material of the production neutralization process, and about 70% of the material is rearranged ester, so the pre-treatment The method is as follows: take 1.0258g of the sample, put it into a 100mL volumetric flask, add mobile phase to constant volume and shake well as the sample stock solution, then remove 1mL of the sample stock solution, dilute it to volume with mobile phase in a 100mL volumetric flask, and use a 0.22μm filter membrane Analysis after filtration.
[0094] The present embodiment analyzes chromatogram such as Figure 4 According to this method, the content of rearranged esters is 69.511%.
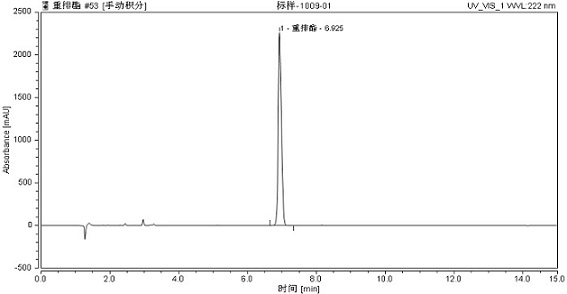
[0095] Among them, in Figure 2-Figure 4 The vertical axis represents absorbance or absorbance, in Figure 2-Figure 4 Expressed in Absorbance.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Concentration | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com