Furfural-based organic photochromic material and preparation method thereof
A photochromic material and organic technology, applied in the direction of color-changing fluorescent materials, organic chemical methods, organic chemistry, etc., can solve the problems of low yield, harsh reaction conditions, rare raw materials, etc., achieve high molar absorptivity, easy to obtain, High fatigue resistance effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
[0053] Compound I of the present invention has a molecular structure as follows:
[0054]
[0055] The preparation method of the compound of this example 1 may further comprise the steps:
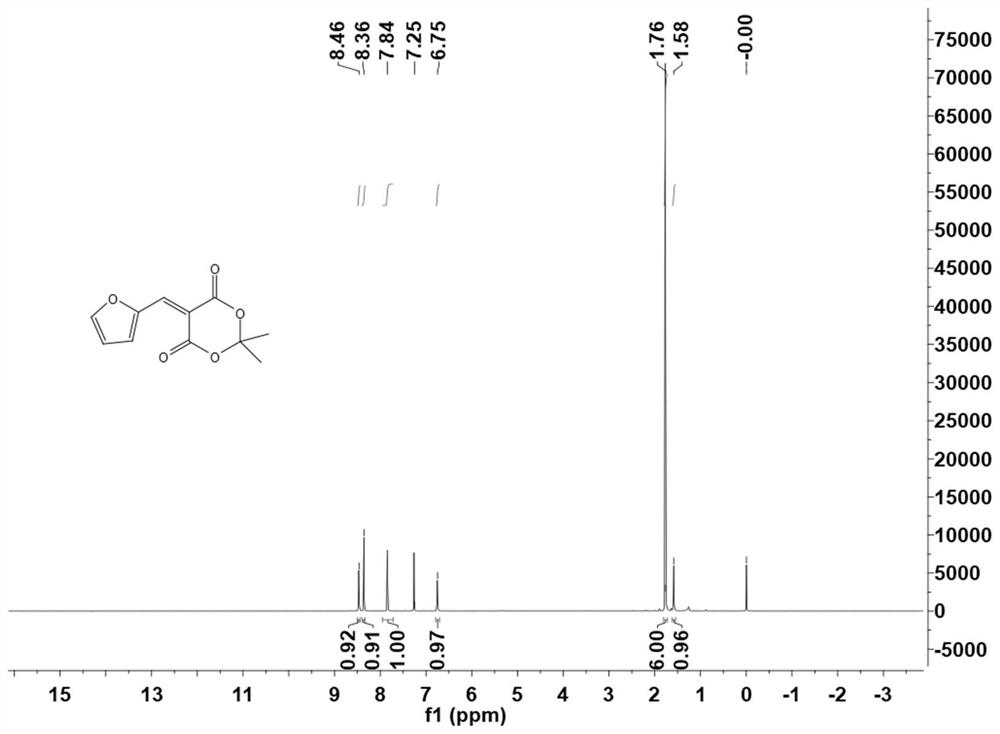
[0056] Cyclo(ethylene)isopropyl malonate and furfural were sequentially added to H 2O middle. The heterogeneous mixture was heated to 75°C and stirred at this temperature for 2 hours, during which time a yellow precipitate formed. After the reaction (TLC) detection, hexane:ethyl acetate=3:1) the mixture was cooled to room temperature. The precipitated solid was collected by vacuum filtration and washed twice with cold water. The collected solid was dissolved in dichloromethane and washed with saturated NaHSO 3 、H 2 O, saturated NaHCO 3 and washed with brine. MgSO for organic layer 4 After drying and filtration, the solvent was removed by rotary evaporator to obtain a bright yellow powder. Its H NMR spectrum is shown in figure 1 .
example 2
[0058] Compound II, its molecular structure is as follows:
[0059]
[0060] The preparation method of the compound of this example 2 may further comprise the steps:
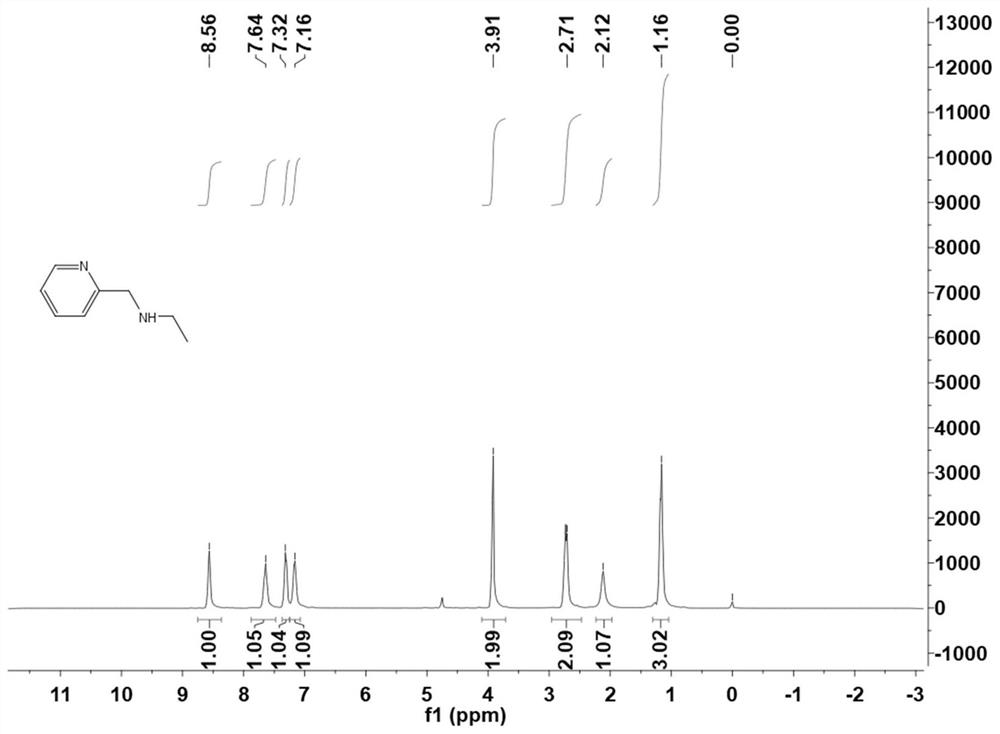
[0061] Mix 2-pyridinecarbaldehyde and ethylamine in a molar ratio of 1:2 and add to methanol, reflux and stir for 2h, then stir at room temperature for another 2h, then extract the yellow reaction mixture with ether three times. anhydrous MgSO 4 Dry, filter and evaporate to dryness, then add NaBH 4 After reduction, a yellow oily liquid was finally obtained. Its H NMR spectrum is shown in figure 2 .
example 3
[0063] Compound III, its molecular structure is as follows:
[0064]
[0065] The preparation method of the compound of this example 3 comprises the following steps:
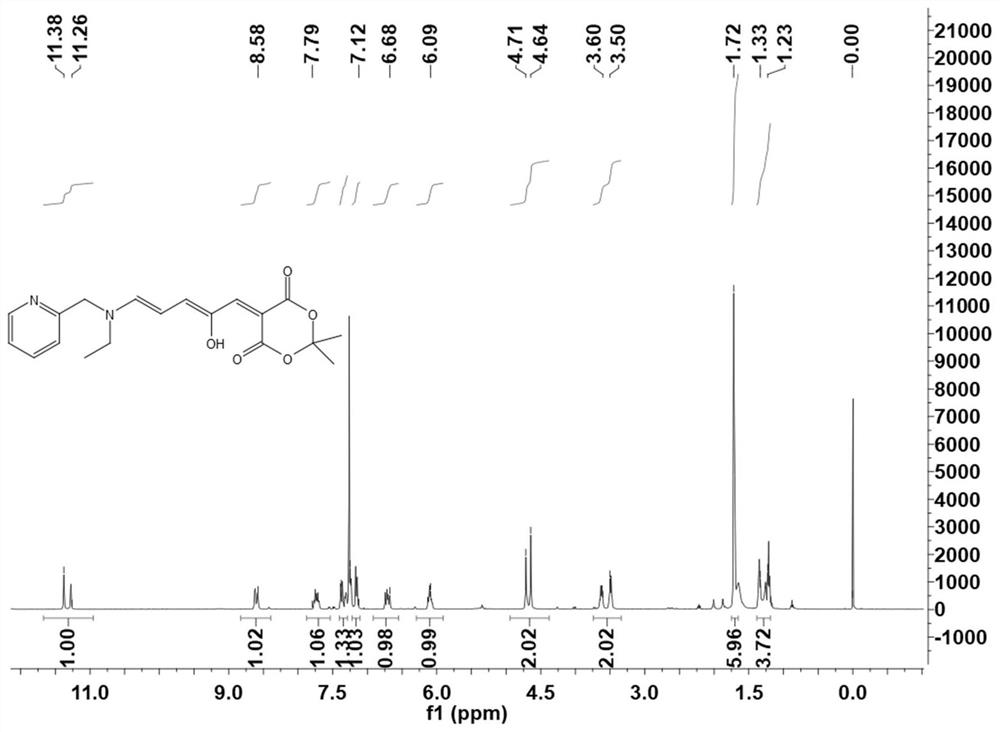
[0066] In the two-necked flask, according to the molar ratio of compound I: pyridineethylamine = 1:1, sequentially add THF. The mixture was stirred for 10 minutes at 23°C, then cooled at 0°C for 20 minutes. After the reaction was complete, the mixture was filtered to collect solids. The solid was washed with cold ether and dried in vacuo to obtain a red solid. Its H NMR spectrum is shown in Figure 3-4 .
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com