Preparation method of L-erythro biopterin compound
A biopterin and compound technology, which is applied in drug combination, organic chemistry, metabolic diseases, etc., can solve the problems of complex operation, difficult purification, expensive raw materials, etc., and achieve the effects of reducing environmental pollution, shortening reaction routes, and reducing production costs
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
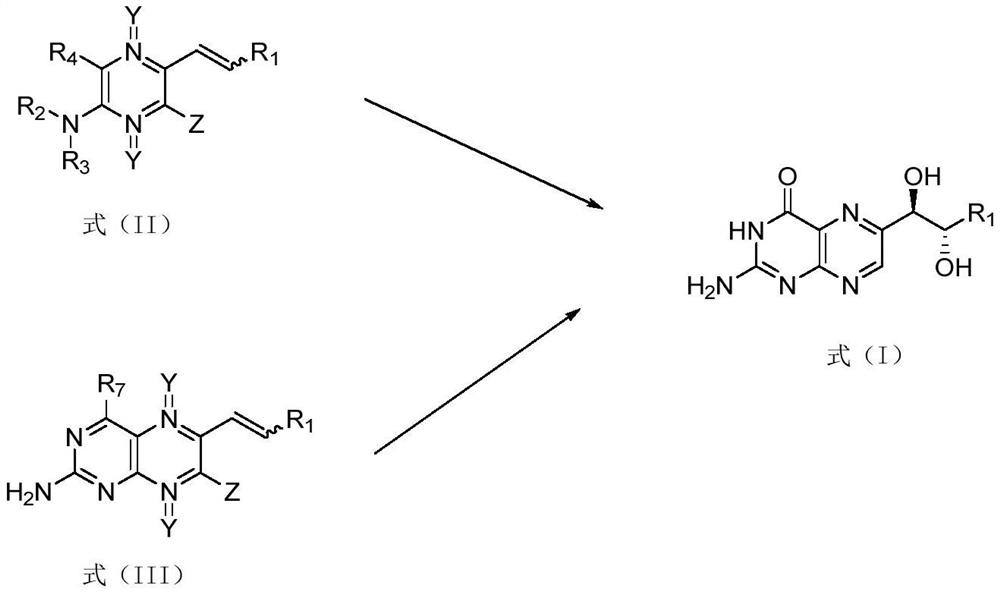
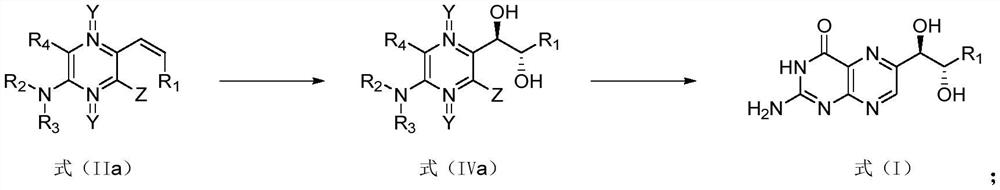
[0192] Wherein, the definition of each substituent is as above, and will not be repeated here. The preparation method of L-erythrotype biopterin compound shown in formula (I) comprises the following steps:
[0193] (1) compound shown in formula (VI) and Carry out Sonogashira reaction, make the compound shown in formula (V);
[0194] (2) compound shown in formula (V) is through catalytic hydrogenation, makes the compound shown in formula (IIa);
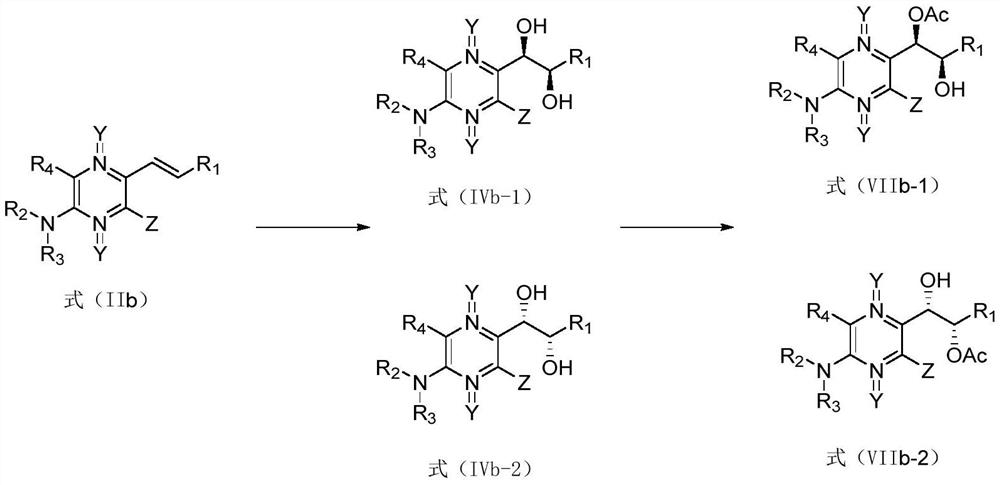
[0195] (3) The compound shown in formula (IIa) is subjected to dihydroxylation reaction to obtain the compound shown in formula (IV);
[0196] (4) compound shown in formula (IV) is through cyclization reaction, makes the compound shown in formula (I-1), and the compound shown in formula (I-1) is hydrolyzed, makes the L- shown in formula (I) Erythro-biopterin compounds.
[0197] In the above (1)-(4), the specific introduction of each reaction is as above, and will not be repeated here.
[0198] understandable when R 4 for-COOR 5...
Embodiment 1
[0222]
[0223] Weigh 200mg compound 8, 11mg CuI, 10mg PdCl 2 , 30mg PPh 3 Place in a 25mL three-neck flask, and add 5mL of acetonitrile. 0.7 mL of triethylamine and 1.1 mL of propyne (1M in THF) were added under stirring at room temperature, and the reaction was stirred for 16 h. 10 mL of water was added to quench the reaction, the layers were separated, and the organic layer was dried and concentrated to obtain 163 mg of crude compound 6, which was used in the next reaction. IR (cm -1 )ν3400, 2226, 1647, 1487, 1192; 1 H NMR (400MHz, DMSO-d 6 )δ8.26(s,1H),7.54(s,2H),2.01(s,3H), 13 C NMR (101MHz, DMSO) δ155.61, 150.33, 128.08, 115.71, 111.16, 88.27, 76.62, 4.18. HRMS m / z (ESI+) C 8 h 7 N 4 + requires: 159.0667; found: 159.0671.
[0224]
[0225] Add 62mg Na to 10mL MeOH and stir until complete reaction, then add 226mg guanidine hydrochloride N 2 Protected, stirred at room temperature for 5min. The insoluble matter in the system was filtered, and 163 mg of com...
Embodiment 2
[0240]
[0241] Disperse 200mg of compound 5 in 5mL of an aqueous solution containing 50mg of NaOH, heat to 78°C, stir for 1h, add acetic acid dropwise to neutralize the system to pH=5-6, filter the precipitated solid, and wash with methanol to obtain compound 5-1 (152mg, purity 98%, yield 76%);
[0242] Dissolve 152mg of compound 5-1 in a combined solution of 5mL MeOH / DCM=1 / 1, stir well to dissolve, add 100mg of Lindlar Pd, replace H 2 (1 atm) stirred at room temperature for 3 days. After filtering the catalyst, the solvent was concentrated to obtain compound 3 (150mg, purity 95%, yield 93%); 1 H NMR (500MHz, DMSO) δ12.3(s, 1H), 8.53(s, 1H), 6.49(s, 2H), 2.04(s, 3H). 13 C NMR (125MHz, DMSO) δ161.85, 156.13, 150.79, 148.55, 136.37, 127.07, 92.12, 85.24, 4.59. HRMS m / z (ESI+) C 9 h 10 N 5 o + requires:203.2050,found:203.2051
[0243]
[0244] Compound 3 is subjected to a dihydroxylation reaction, including the following methods:
[0245] 1) Sharpless asymmetric di...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com