Hypoallergenic infant formula goat milk powder
An infant formula, hypoallergenic technology, applied to bacteria, whey, dairy products, etc. used in food preparation, can solve problems such as inability to continue breastfeeding infants, to avoid the occurrence of neonatal bovine milk protein allergy and prevent infection , the effect of enhancing immunity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1-6
[0022] Example 1-6 Hypoallergenic infant formula goat milk powder
[0023] Examples 1-6 are respectively a method for preparing a hypoallergenic infant formula goat milk powder, see Table 1 for the raw material composition of the milk powder, and see Table 2 for the process parameters of the milk powder.
[0024] The preparation method is carried out according to the following steps:
[0025] S1. Standardize raw goat milk, separate impurities, and pasteurize at 70-75°C for 15-18s to prepare goat milk. Add high-oil desalted goat whey powder, lactose, and goat whey protein powder to goat milk , soybean oil, 1,3 dioleic acid-2-palmitic acid triglyceride, linseed oil, galacto-oligosaccharides, anhydrous butter, fructo-oligosaccharides, complex minerals, multivitamins, calcium carbonate, potassium hydroxide, Choline chloride and phospholipids are mixed and fully dissolved to obtain material A;
[0026] S2. Material A is preheated to 58-62°C, then homogenized under a pressure of 1...
Embodiment 7
[0035] Example 7 In vitro cell model evaluation
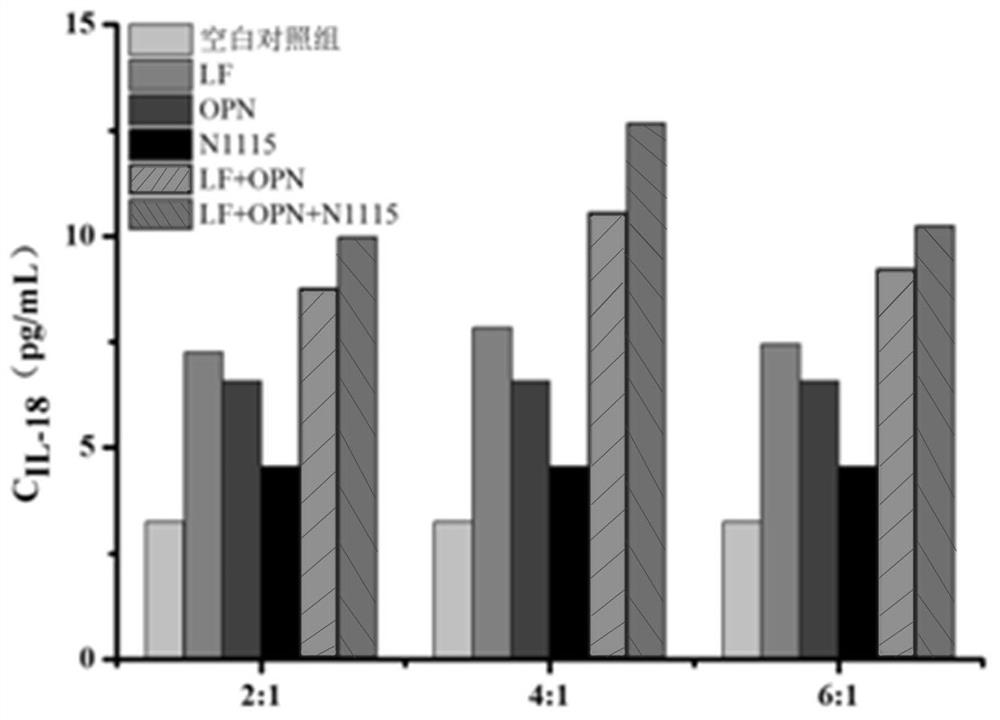
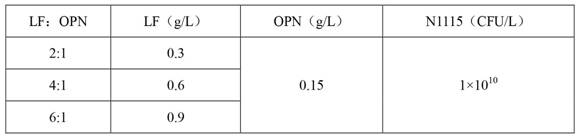
[0036] In this example, different addition ratios of lactoferrin (LF) and osteopontin (OPN) are set to compound Lactobacillus paracasei N1115, and the superior ratio of the above ingredients is evaluated through an in vitro cell model. The specific evaluation method is as follows:
[0037] Caco-2 cells were cultured on serum-free medium for 2 hours, and then lactoferrin (LF), osteopontin (OPN) and Lactobacillus paracasei N1115 were added respectively, and LF-OPN mixtures with different concentration ratios were compounded with paracasein Bacillus N1115 (the specific addition concentration is shown in Table 3, diluted 20 times) on the cell culture medium containing 1% fetal bovine serum, and incubated at 37°C for 72 hours. The cell culture medium was collected, concentrated through an ultrafiltration centrifuge tube, and the content of IL-18 was determined using an ELISA kit.
[0038] Table 3 Mixture Composition
[0039]
...
Embodiment 8
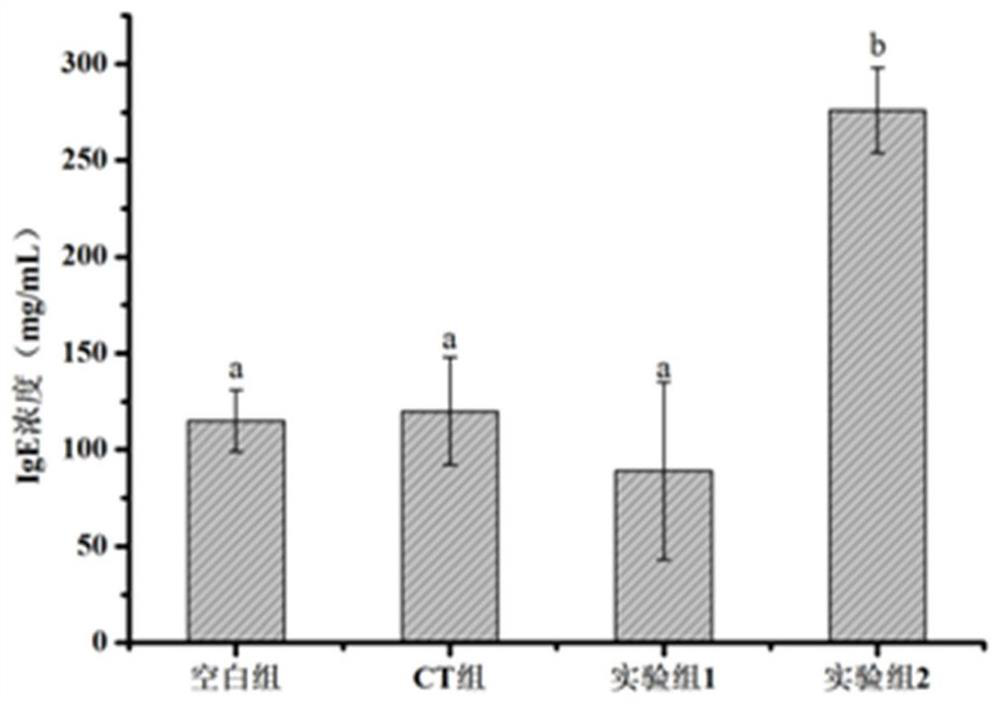
[0041] Example 8 Allergenicity test of hypoallergenic infant formula goat milk powder
[0042]Forty male mice were selected and randomly divided into 4 groups, namely blank group, CT group, experimental group 1 and experimental group 2, with 10 mice in each group. The culture period was 6 weeks, and they were administered orally once a week. Among them, the blank group did not receive any treatment, the CT group only received 10 μg of vibrio cholerae toxin (CT) per stomach, and the first 5 times of oral administration of the low-sensitivity infant formula goat milk powder of Example 1 in the experimental group 1 was 20 mg per child. , and supplemented with Vibrio cholerae toxin (CT) 10 μg / mouse, in order to prevent the mice from developing immune tolerance, the amount of the low-sensitivity infant formula goat milk powder of the last gavage embodiment 1 was 50 mg / mouse, and the experimental group 2 The first 5 gavages of ordinary skimmed milk powder were 20 mg / mouse, supplemen...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com