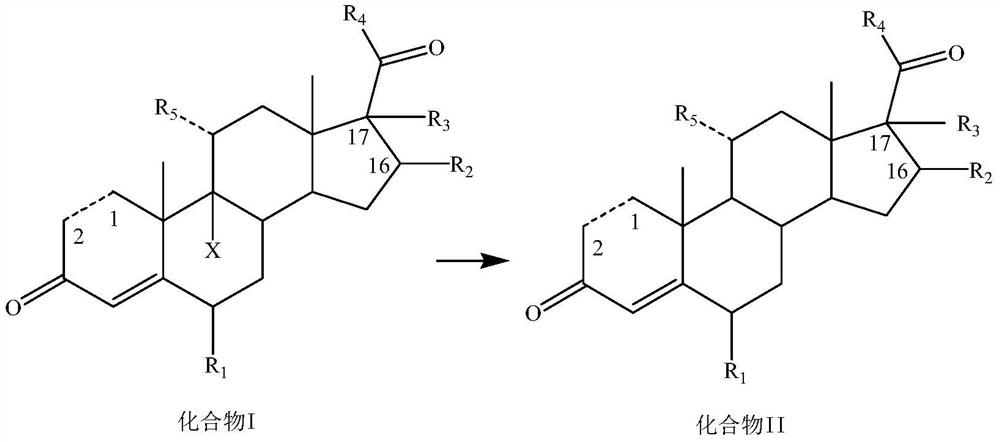
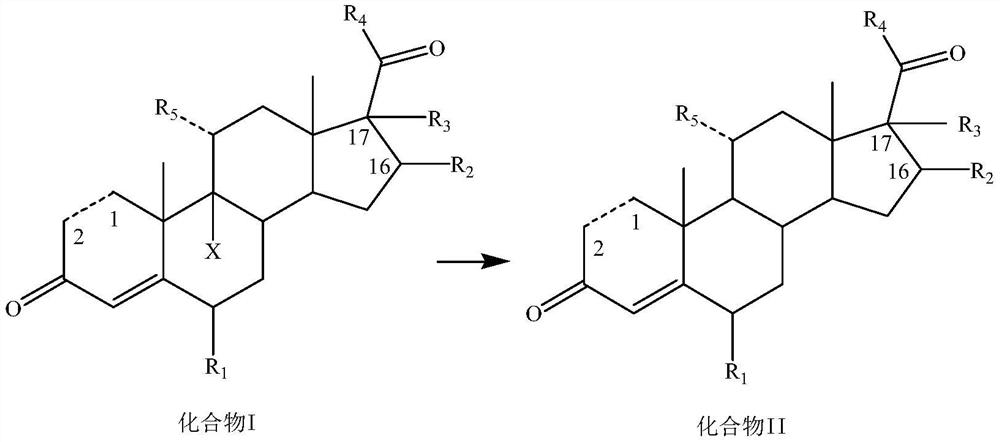
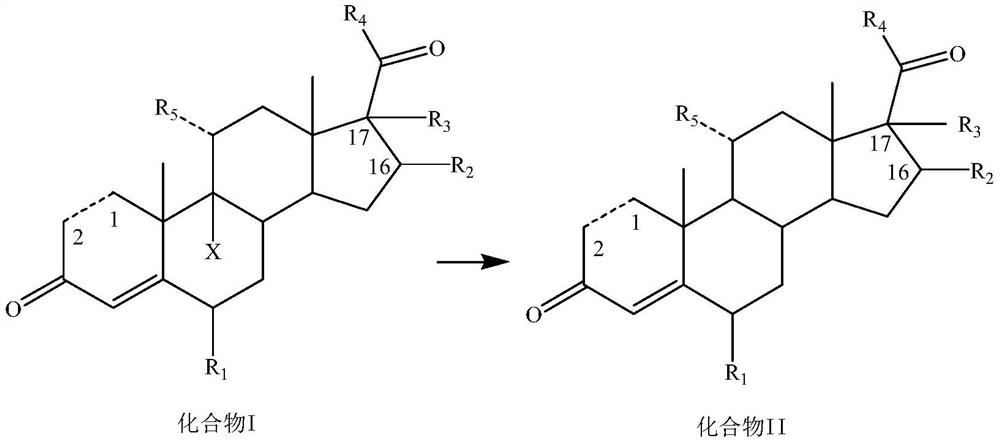
Dehalogenation methodof 9-halogenated steroid compound and application
A technology of steroidal compounds and compounds, applied in the direction of steroidal compounds, organic chemistry, etc., can solve problems affecting normal production of products, high cost of environmental protection treatment, environmental pollution, etc., achieve simple and easy synthesis process, improve production applicability, highly operable effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0141]
[0142] Add 200mL of 75% ethanol, 9.8g of potassium carbonate, 11mL of 50% aqueous hypophosphorous acid, 20g of compound I-1 and 4.3g of AIBI to the reaction flask in turn at room temperature, heat up to 70°C for reaction, TLC until there is no raw material, and post-treatment to precipitate a solid , filtered, and dried to obtain 15.2 g of compound II-1 as a solid, with a yield of 92.3% and a purity of 96.8%.
Embodiment 2
[0144]
[0145] Add 200mL 2-methyltetrahydrofuran, 9g sodium carbonate, 9.9mL 50% hypophosphorous acid aqueous solution, 20g compound I-2 and 3.3g AIBA to the reaction flask successively at room temperature, heat up to 65°C for reaction, TLC until there is no raw material, post-treatment The solid was precipitated, filtered, and dried to obtain 15.2 g of compound II-2 as a solid, with a yield of 91.5% and a purity of 96.5%.
Embodiment 3
[0147]
[0148] Add 160mL of n-butanol, 13mL of 33% sodium hydroxide solution, 9.9mL of 50% hypophosphorous acid aqueous solution, 20g of compound I-3 and 2.5g of CABN into the reaction flask successively at room temperature, raise the temperature to 80°C for reaction, TLC until there is no raw material, After post-treatment, a solid was precipitated, filtered, and dried to obtain 15.3 g of compound II-3 as a solid, with a yield of 93.4% and a purity of 97.0%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com