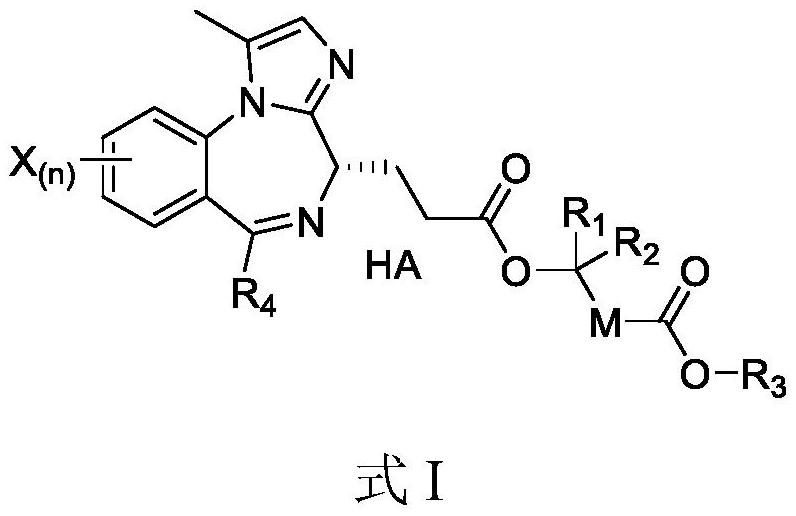
Benzodiazepine compound as well as preparation method and pharmaceutical effect thereof
A compound and composition technology, applied in the field of chemical medicine, can solve the problems of by-product toxicity, high blood drug concentration, affecting the activity of drug metabolizing enzymes, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0167] Embodiment 1, the preparation of intermediate 1 of the present invention
[0168]
[0169] At -40°C, add 5.25ml (29.83mmol, 4.4eq) 2-bromopyridine to a three-necked flask containing 20ml (27.12mmol, 4.0eq) n-butyllithium (2.5M) and 40ml anhydrous ether, and stir for 1 hour , and then added dropwise a solution containing 2.0g (6.78mmol, 1.0eq) of 2-amino-4,5-dibromobenzoic acid in 30ml of tetrahydrofuran, and reacted at 0°C for 3 hours. After the reaction of the raw materials was basically complete, ice water was added, extracted with ethyl acetate, dried and filtered over anhydrous sodium sulfate, concentrated under reduced pressure to obtain an oil, and 2.17 g of a yellow solid (Intermediate 1) was obtained by column chromatography.
[0170] The H NMR spectrum (deuterated chloroform) of the intermediate 1: δ8.78(d,1H),8.43(d,1H),8.07(d,1H),7.96–7.82(m,2H),6.87(d ,1H). MS: m / z: 355.90 (M+1).
Embodiment 2
[0171] Embodiment 2, the preparation of intermediate 2 of the present invention
[0172]
[0173] 2.0g (5.62mol, 1.0eq) 2-amino-4,5-dibromophenyl-pyridin-2-yl-methanone and 2.8g (10.72mmol, 1.9eq) (R)-2-tert-butoxy Carbonyl-amino-5-methoxy-5-oxopentanoic acid was dissolved in 30mL of dichloromethane (DCM), and 2.2g (10.68mmol, 1.9eq) of DCC (dicyclohexylcarbodiimide ), react overnight at room temperature. Point the board, and the raw materials basically react completely. The reaction solution was filtered and concentrated under reduced pressure, and 3.3 g of a light yellow oily substance (Intermediate 2) was obtained by column chromatography.
[0174] The H NMR spectrum of the intermediate 2 (deuterated chloroform): δ11.36(s, 1H), 8.74(d, J=4.8Hz, 1H), 8.57(d, J=9.0Hz, 1H), 7.99– 7.92(m,3H),7.67(dd,J=9.0,2.2Hz,1H),7.56–7.51(m,1H),5.42(d,J=6.6Hz,1H),4.35(s,1H),3.68 (s, 3H), 2.54–2.24 (m, 4H), 1.44 (s, 9H). MS: m / z: 600.28 (M+1).
Embodiment 3
[0175] Embodiment 3, the preparation of intermediate 3 of the present invention
[0176]
[0177] 3.3g (R)-4-((tert-butoxycarbonyl)amino)-5-((4,5-dibromo-2-pyridylphenyl)amino)-5-oxopentanoic acid methyl ester Dissolve in 20mL methanol, add 20mL HCl / MeOH solution (the volume ratio of HCl and MeOH is 1:1), and react overnight at room temperature. Point the board, and the raw materials basically react completely. Use directly in the next step without treatment.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com