Green fluorescent diamine-oxazoline zinc complex as well as preparation method and application thereof
An oxazoline zinc, green fluorescence technology, applied in chemical instruments and methods, luminescent materials, organic chemistry and other directions, can solve the problems of high price, difficult mining, fluorescence lifetime and narrow emission spectrum, and achieve high yield , good stability, high product purity effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
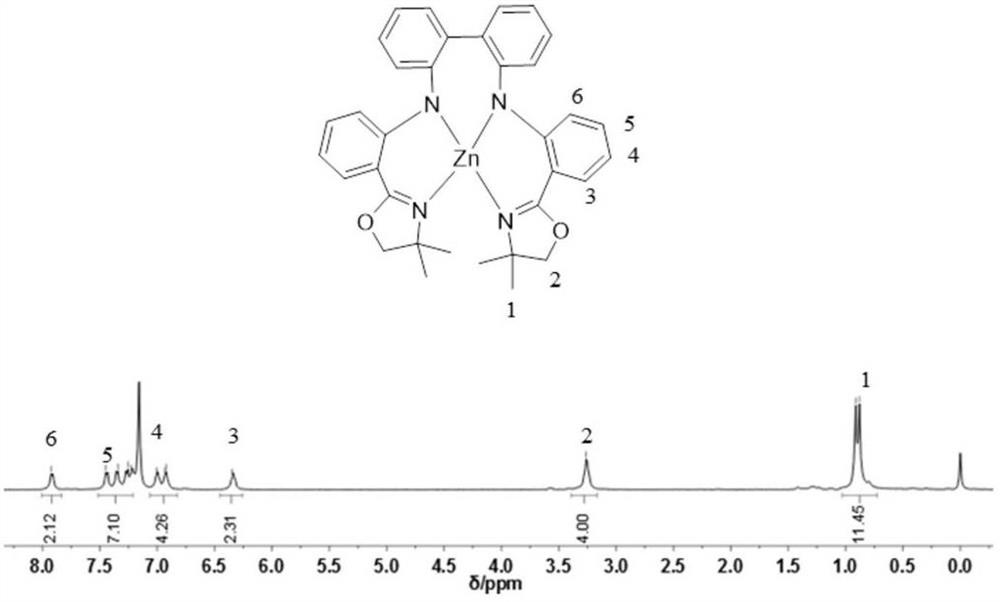
[0027] L 1 Preparation of -Zn complexes (L 1 represents a methyl-substituted bisamine-oxazoline ligand)
[0028]
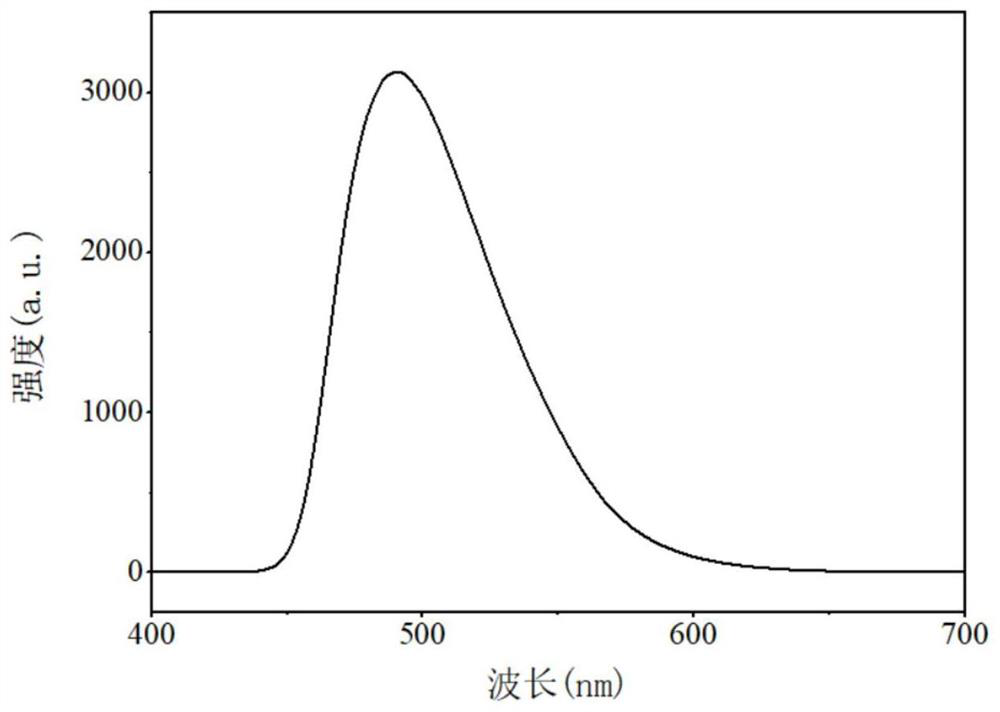
[0029] Under nitrogen protection, diethylzinc (0.415ml, 0.415mmol) and bisamine-oxazoline ligand H 2 L 1 (0.2g, 0.377mmol) were dissolved in 5ml tetrahydrofuran, diethylzinc solution was added dropwise to the ligand solution at -78°C, slowly raised to room temperature and reacted for 12h, and the solvent was removed under vacuum to obtain L 1 -Zn complex 0.210g, yield 95.6%. L 1 -Zn complex H NMR spectrum such as figure 1 shown. L 1 -Zn complex fluorescence spectrum as shown in figure 2 shown.
Embodiment 2
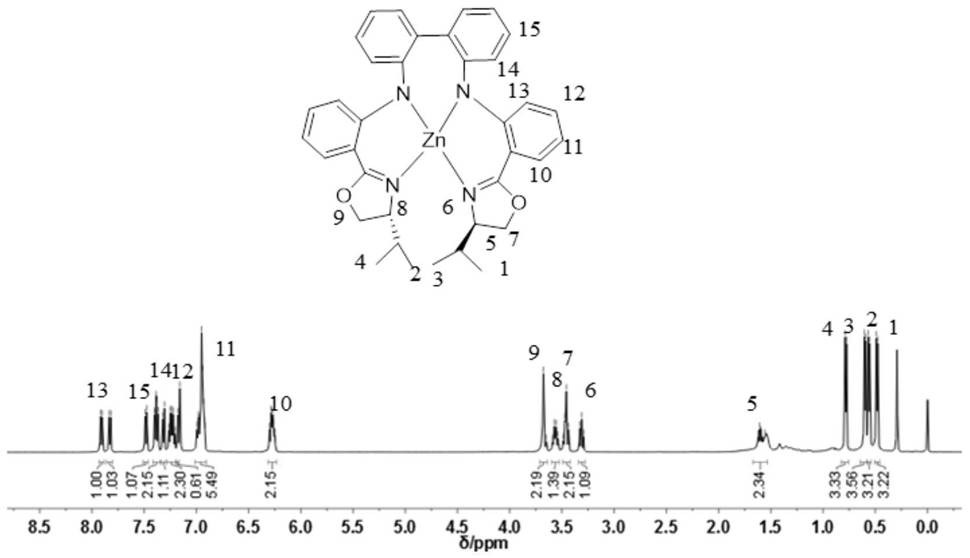
[0031] L 2 Preparation of -Zn complexes (L 2 represents an isopropyl-substituted bisamine-oxazoline ligand)
[0032] The preparation process of the complex is the same as the preparation method in Example 1, and the preparation method of the complex is as follows:
[0033]
[0034] The specific steps are similar to the method in Example 1, except that the ligand H 2 L 2 Replaced Ligand H above 2 L 1 . get L 2 -Zn complex 0.203g, yield 91.9%. L 2 -Zn complex H NMR spectrum such as image 3 shown. L 2 -Zn complex UV spectrum as shown in Figure 4 shown.
Embodiment 3
[0036] L 3 Preparation of -Zn complexes (L 3 represents a phenyl-substituted bisamine-oxazoline ligand)
[0037] The preparation process of the complex is the same as the preparation method in Example 1, and the preparation method of the complex is as follows:
[0038]
[0039] The specific steps are similar to the method in Example 1, except that the ligand H 2 L 3 Replaced Ligand H above 2 L 1 . get L 3 -Zn complex 0.195g, yield 88.9%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com