Method for simply, conveniently and controllably synthesizing alpha-sulfydryl-omega-hydroxyl polyether by taking thiocarboxylic acid as initiator
A technology of thiocarboxylic acid and hydroxyl polyether, which is applied in the field of polyether synthesis, can solve the problems of slow development of synthetic methods, and achieve the effect of strong nucleophilicity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0052] One-pot two-step synthesis of α-mercapto-ω-hydroxypolyethylene oxide. (1) Ring-opening polymerization of ethylene oxide (EO) was carried out using thioacetic acid as an initiator and a metal-free Lewis acid-base pair as a catalyst; (2) In situ aminolysis deacetylation. The specific operation is as follows:
[0053] Both tetrahydrofuran (THF) and EO are used after purification and dehydration treatment, and thioacetic acid can be used directly. Under an inert atmosphere, mix 1 part of thioacetic acid (0.5 mmol), 0.1 part of phosphazene base t BuP 1 , 0.5 parts of tri-sec-butylborane and THF (1ml) were successively added into a dry glass reactor and stirred and mixed evenly. In this example, the molar ratio of thioacetic acid, organic base and alkylboron was 1:0.1:0.5 . Connect the glass reactor to a vacuum line, remove part of the gas in the bottle, and cool down with an ice-water bath. Distilled 90 parts of dry EO (where [EO] 0 =15mol / L), and react in a sealed gla...
Embodiment 2
[0056]Two-pot synthesis of α-mercapto-ω-hydroxypolyethylene oxide. (1) With thioacetic acid as initiator, metal-free Lewis acid-base pair is the ring-opening polymerization of ethylene oxide (EO) implemented as a catalyst; (2) the α-thioacetate-ω that the first step obtains After the -hydroxypolyethylene oxide precipitate is dried, the acetyl group is deacetylated by ammonia cleavage. The specific operation is as follows:
[0057] The first step epoxy polymerization is with embodiment 1. After the end of the polymerization reaction, the product was collected, diluted with THF (2mL) and added with neutral Al 2 o 3 Stir, filter twice, and vacuum dry to obtain α-thioacetate-ω-hydroxypolyethylene oxide. Under an inert atmosphere, weigh the initial product in a clean glass reactor, add 7.0 mol / L ammonia in methanol solution (the amount of ammonia used is 10 parts) and methanol (1 mL), and react at room temperature for 8 h. After the reaction was completed, the vacuum line was ...
Embodiment 3
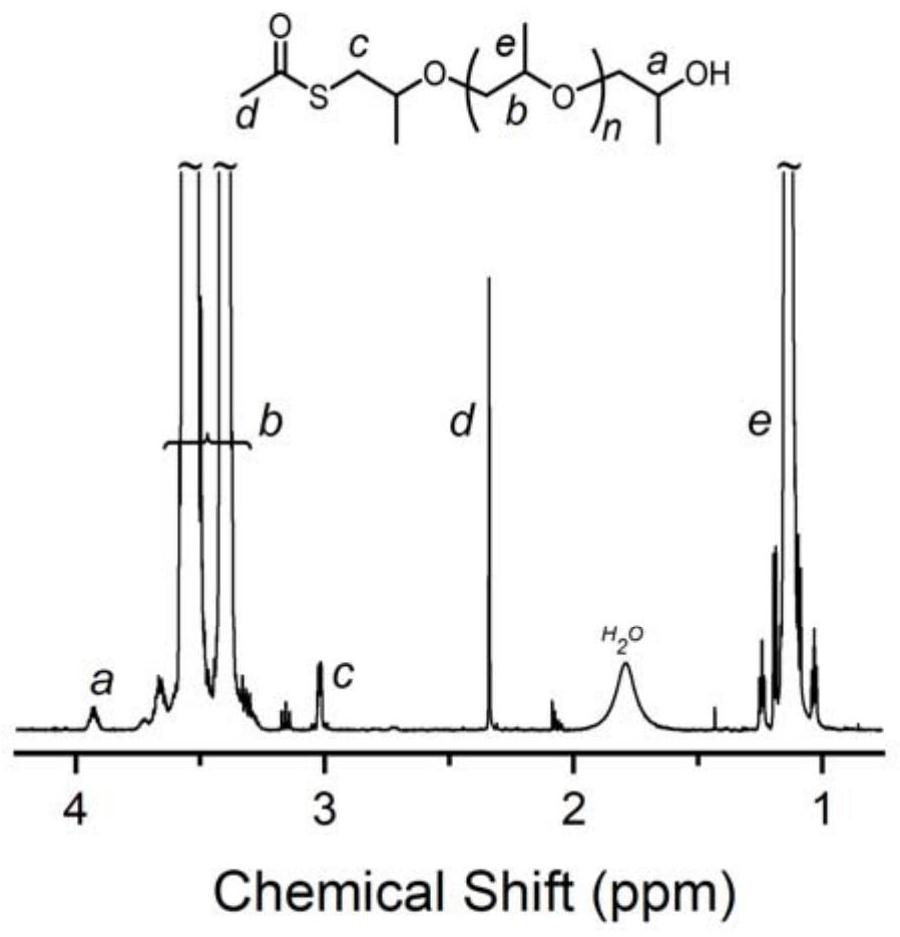
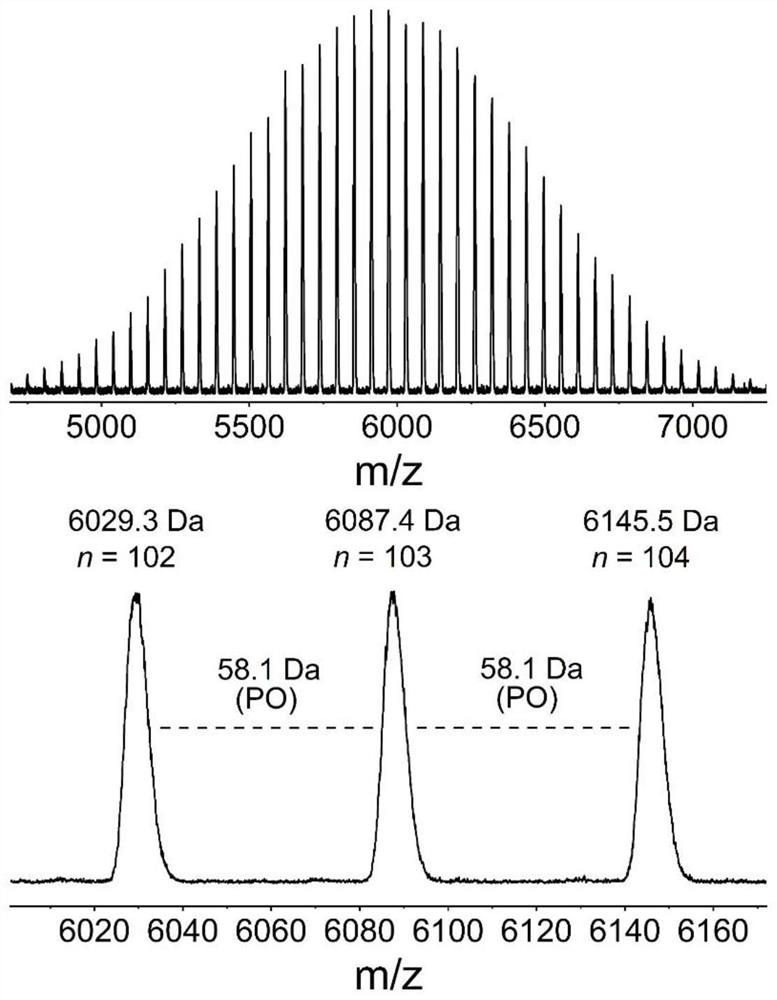
[0059] Change the amount of Lewis acid-base pair, add 0.01 part of phosphazene base t BuP 1 And 0.01 part of tri-sec-butylborane, the rest are consistent with Example 1, and the EO polymerization time is extended to 6h. In this example, the molar ratio of thioacetic acid, organic base and alkyl boron is 1:0.01:0.01. After the first polymerization reaction is completed, 1 The conversion rate of EO monomer was 100% as measured by H NMR, the molecular weight of the crude product as measured by SEC was 4.3 kg / mol, and the degree of dispersion was 1.10. 1 H NMR (600MHz, Chloroform-d), δ3.92 (ddd, J=9.1, 6.3, 2.8Hz, 2H), 3.65 (m, 366H), 3.02 (dq, J=5.1, 1.8Hz, 2H), 2.34 (d,J=1.3Hz,3H).
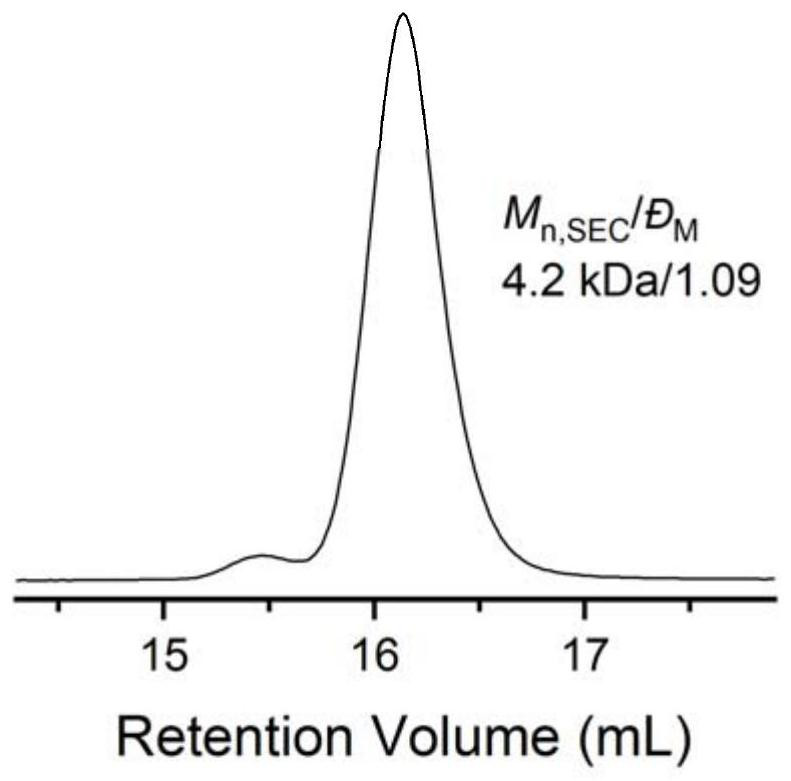
[0060] After the second step ammonolysis reaction finishes, 1 The degree of thiol functionalization as measured by H NMR is 100%, the molecular weight of the product as measured by SEC is 4.2 kg / mol, and the degree of dispersion is 1.09. 1 H NMR (600MHz, Chloroform-d), δ3.93 (d, J=6.4Hz, 2H), ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Molecular weight | aaaaa | aaaaa |
| Molecular weight | aaaaa | aaaaa |
| Molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com