A kind of chiral 2,3-dihydrobenzofuran derivative and its preparation method
A technology of furan derivatives and dihydrobenzene, applied in the field of chiral 2,3-dihydrobenzofuran derivatives and its preparation, achieving the effects of environmental friendliness, convenient operation and high enantioselectivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
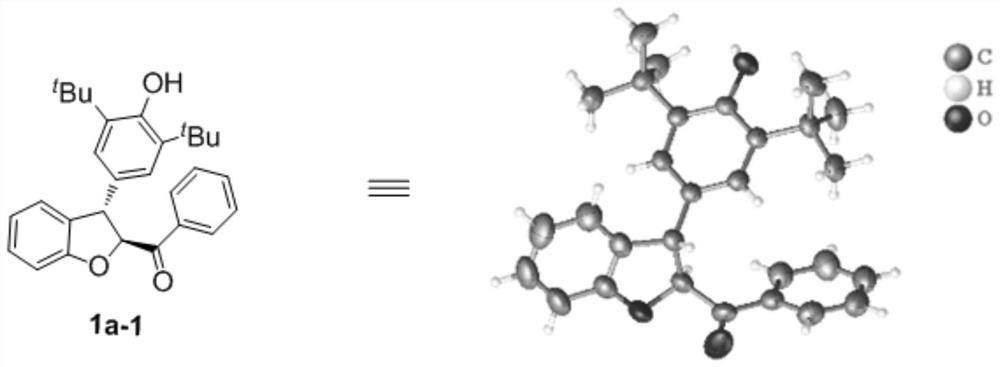
[0053] ((2s,3s)-3-(3,5-di-tert-butyl-4-hydroxyphenyl)-2,3-dihydrobenzofuran-2-yl)(phenyl)methanone (1a-1 ) preparation:
[0054]
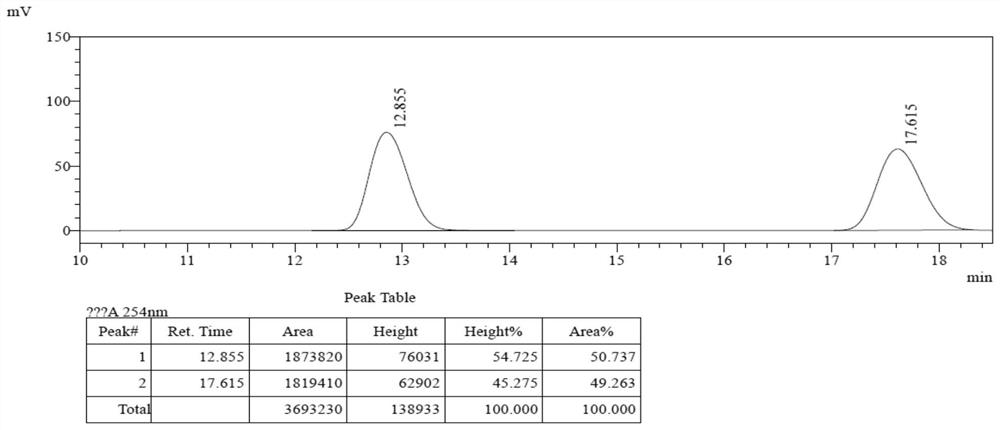
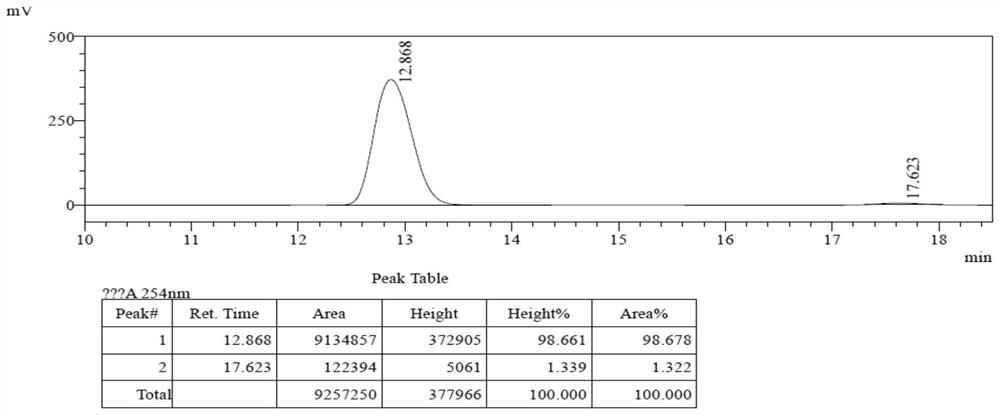
[0055] Add 31.0mg of compound 2a (0.1mmol) and 24mg of compound 3a (0.12mmol), 15.2mg (0.15mmol) of triethylamine, catalyst 4d (0.01mmol) and 1mL of acetonitrile into the reaction flask, stir and mix well at 30°C The reaction occurs below. The reaction catalyst loading was 5mol%, and the reaction was 6h. TLC showed that the raw material 2a was completely consumed, and direct concentration column chromatography (petroleum ether / ethyl acetate, v / v=40 / 1) gave 46.8 mg of product 1a-1, which Single crystal structure see figure 1 ; Yield 96%, 97%ee, >99:1dr.
[0056] NMR and MS data: 1 H NMR (400MHz, CDCl 3 )δ7.93(d, J=7.2Hz, 2H), 7.59(dd, J=7.6, 7.2Hz, 1H), 7.44(dd, J=8.0, 7.6Hz, 2H), 7.20(dd, J=8.0 ,7.6Hz,1H),7.04(d,J=7.2Hz,1H),6.98(d,J=8.0Hz,1H),6.94(s,2H),6.90(dd,J=7.6,7.2Hz,1H ), 5.77(d, J=6.4Hz, 1H), 5.17(s, 1H), 4.85(d, J=6.4Hz, 1H), 1.3...
Embodiment 2
[0058] ((2s,3s)-3-(3,5-di-tert-butyl-4-hydroxyphenyl)-6-methyl-2,3-dihydrobenzofuran-2-yl)(phenyl)methyl Preparation of ketone (1a-2):
[0059]
[0060] Add 32.4mg of compound 2b (0.1mmol) and 24mg of compound 3a (0.12mmol), 15.2mg (0.15mmol) of triethylamine, catalyst 4e (0.01mmol) and 1mL of acetonitrile into the reaction flask, stir and mix with 30°C A [4+1] cycloaddition reaction occurs. The reaction catalyst loading is 5mol%, reacted for 4h, TLC showed that the raw material 2b was completely consumed, and directly concentrated column chromatography (petroleum ether / ethyl acetate, v / v=30 / 1) to obtain 45.0 mg of product 1a-2. Rate 93%, 99% ee, >99:1dr.
[0061] NMR and MS data: 1 H NMR (400MHz, CDCl 3 )δ7.91(d, J=7.2Hz, 2H), 7.58(dd, J=7.6, 7.2Hz, 1H), 7.43(dd, J=8.0, 7.6Hz, 2H), 6.99(d, J=7.6 Hz,1H),6.95(s,2H),6.86(d,J=8.4Hz,2H),5.75(d,J=6.8Hz,1H),5.17(s,1H),4.79(d,J=6.8 Hz,1H),2.24(s,3H),1.39(s,18H); 13 C NMR (101MHz, CDCl 3 )δ195.50,157.29,153.17,136.38,134.77...
Embodiment 3
[0063] ((2s 3s)-3-(3,5-di-tert-butyl-4-hydroxyphenyl)-7fluoro-2,3-dihydrobenzofuran-2-yl)(phenyl)methanone (1a -3) Preparation:
[0064]
[0065] Add 32.8mg of compound 2c (0.1mmol) and 24mg of compound 3a (0.12mmol), 15.2mg (0.15mmol) of triethylamine, catalyst 4e (0.01mmol) and 1mL of acetonitrile into the reaction flask, stir and mix well with 20°C A [4+1] cycloaddition reaction occurs. The reaction catalyst loading is 5mol%. After 6 hours of reaction, TLC showed that the raw material 2c was completely consumed. Direct concentration and column chromatography (petroleum ether / ethyl acetate, v / v=20 / 1) gave 42.0 mg of product 1a-3. Rate 94%, 99% ee, >99:1dr.
[0066] NMR and MS data: 1 H NMR (400MHz, CDCl 3 )δ7.96(d, J=7.2, Hz, 2H), 7.61(dd, J=7.6, 7.2Hz, 1H), 7.46(dd, J=8.0, 7.6Hz, 2H), 7.03-6.97(m, 1H),6.95(s,2H),6.88-6.80(m,2H),5.86(d,J=6.8Hz,1H),5.20(s,1H),4.95(d,J=6.8Hz,1H), 1.39(s,18H); 13 C NMR (101MHz, CDCl 3 )δ194.43, 153.34, 148.83, 146.38, 145.96 (d, J C...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com