Reaction method for selectively synthesizing aromatic aldehyde or aromatic carboxylic acid
A technology for aromatic carboxylic acids and aromatic aldehydes, which is applied in the preparation of carboxylate salts, chemical instruments and methods, and the preparation of organic compounds. It can solve problems such as lack of atom economy, complicated catalyst preparation, and narrow substrate range. Good conversion rate, achieve repeated use, low price effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
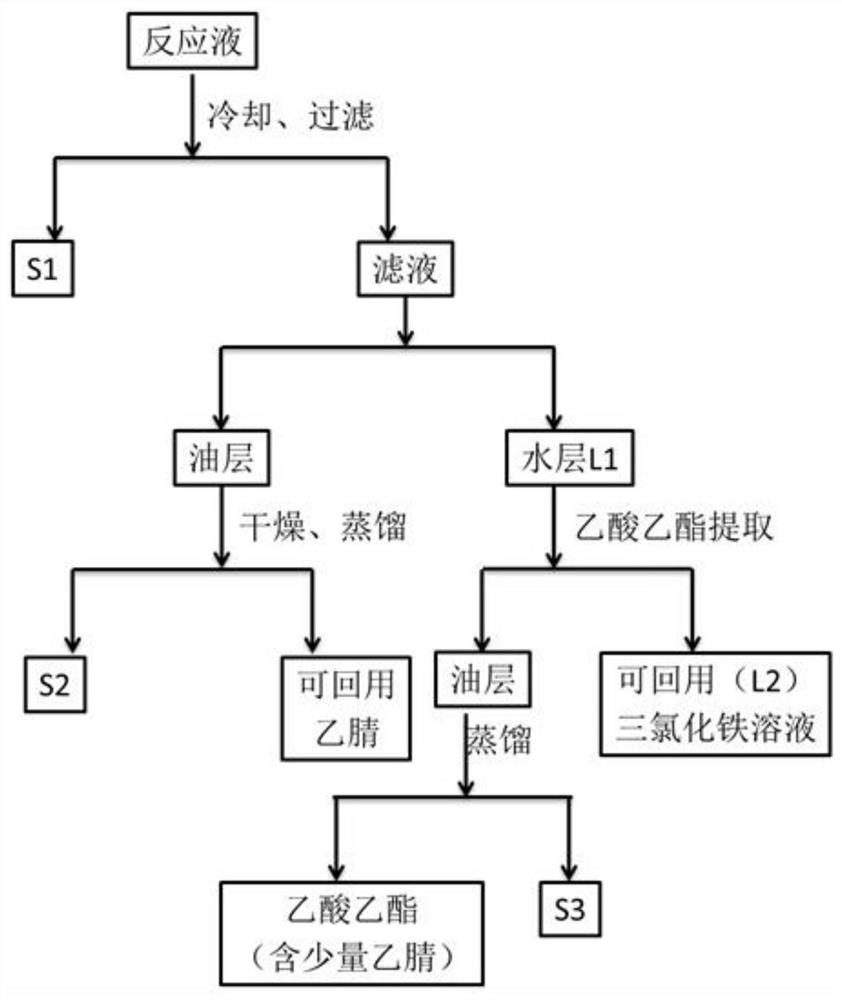
Image
Examples
Embodiment 1
[0021] Embodiment 1: the photocatalytic synthesis of p-chlorobenzaldehyde
[0022] In a 25mL colorless transparent glass test tube, add 0.5mmol p-chlorotoluene, 5mL acetonitrile, 1mmol ferric sulfate, and 5mL water. Air is introduced through the mouth of the test tube. Under the conditions of air cooling and vigorous stirring, the reaction solution was irradiated with a 50W ultraviolet light source with a wavelength of 350-370nm for 10h. Stop the reaction, extract with ethyl acetate (10mL × 3), combine the organic layers, dry with sodium sulfate, and remove the organic solvent by rotary evaporation to obtain a crude product, which is analyzed by normalized area method gas chromatography-mass spectrometry. For: p-chlorobenzyl alcohol 3%, p-chlorobenzaldehyde 90%, p-chlorobenzoic acid 2.5%, p-chlorotoluene 4%.
Embodiment 2
[0023] Embodiment 2: the photocatalytic synthesis of p-chlorobenzoic acid
[0024] In a 25mL colorless transparent glass test tube, add 0.5mmol p-chlorotoluene, 5mL acetonitrile, 0.25mmol ferric sulfate, and 5mL water. Air is introduced through the mouth of the test tube. Under the condition of vigorous stirring, the air was introduced, and the reaction solution was irradiated with a 50W ultraviolet light source with a wavelength of 350 to 370nm, and reacted for 16 hours to obtain a milky white yellowish solid-liquid mixture. The reaction solution was cooled down, extracted with ethyl acetate (10mL×3), the organic layers were combined, dried over sodium sulfate, the organic solvent was removed by rotary evaporation, and purified by column chromatography to obtain 0.071g of p-chlorobenzoic acid, melting point 240°C, yield 81 %.
Embodiment 3
[0025] Embodiment 3: the synthesis of benzaldehyde
[0026]In a 25mL colorless transparent glass test tube, add 0.5mmol toluene, 1mmol ferric sulfate, 5mL water, 5mL acetonitrile, and introduce air through the test tube mouth. The whole reaction system is sealed to prevent low yield due to volatilization of toluene. Under the condition of vigorous stirring, the reaction solution was irradiated with a 50W ultraviolet light source with a wavelength of 350-370nm for 12h. After the reaction, the reaction solution was extracted with dichloromethane (10mL×3), the organic layers were combined, dried over anhydrous sodium sulfate, and then the solvent was removed by rotary evaporation, and separated by column chromatography to obtain 0.041g of benzaldehyde with a yield of 77%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com