Catalyst for catalyzing esterification reaction and synthesis method thereof
A technology for catalyzing esterification and synthesis methods, applied in the field of catalysts for catalyzing esterification reactions and their synthesis, can solve the problems of catalyst loss of activity, etc., and achieve the effects of reduced dosage, environmental friendliness, and good dispersibility
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
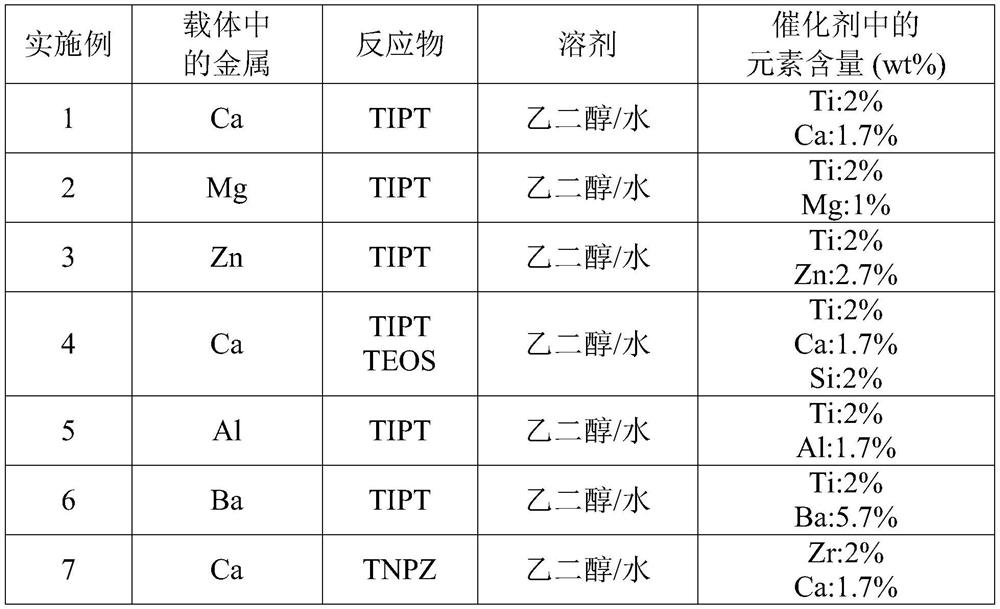
[0027] The disclosure provides a method for synthesizing a catalyst for catalyzing an esterification reaction, comprising the following operations: (1) mixed metal chlorides, metal nitrates, metal carbonates, metal carboxylates, metal One of the sulfate and the oxide of silicon, and the polar solvent form a first mixed solution, wherein the polar solvent includes water, glycol, polyol or a combination thereof. (2) Adding an alkaline substance to the first mixed liquid to form a dispersion liquid. The dispersion contains multiple carriers. For example, the support can be a metal hydroxide, a metal oxide, a metal chloride, a metal nitride, a metal sulfide, a silicon oxide, or a combination thereof. (3) Adding metal alkoxides to the dispersion liquid to form a second mixed liquid, wherein the metal alkoxides include titanate, aluminate, zirconate or a combination thereof. (4) heating the second mixed liquid to form a catalyst. The catalyst includes a polar solvent, multiple su...
experiment example 1
[0032] The following embodiments are used to describe specific aspects of the present invention in detail, and enable those skilled in the art to implement the present invention. However, the following examples should not be used to limit the present invention. Experimental example one: the catalyst of synthesis embodiment 1~7
[0033] The experimental procedure for synthesizing the catalyst of Example 1 is briefly described as follows. To a round bottom flask equipped with a stirrer, 25 g of calcium chloride (225 mmol Ca) and 30 g (1.6 mol) of water were added. Next, 330 g of ethylene glycol (ethyleneglycol) was added to form a first mixed liquid. Then, add 90g of 10wt% sodium hydroxide aqueous solution to form a dispersion liquid containing calcium hydroxide (Ca(OH) 2 ) carrier, the pH of the dispersion is between 9 and 10. Finally, 64 g (225 mmol Ti) of titanium isopropoxide (alkoxide of metal) as a reactant was added to the dispersion to form a second mixed liquid in w...
experiment example 2
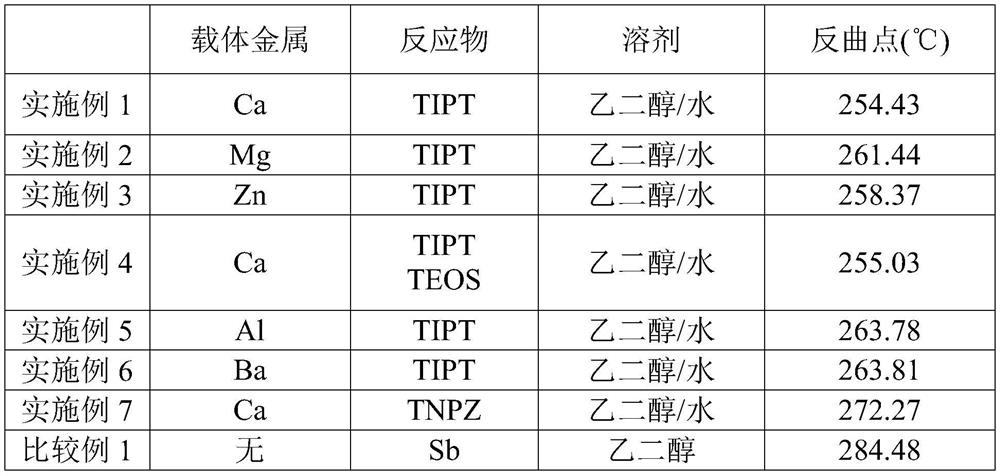
[0037] Experimental Example 2: Performance testing of the catalysts of Examples 1-7 and the antimony acetate of Comparative Example 1
[0038] Performance tests were performed on the catalysts in Examples 1-7 above using a thermogravimetric analyzer / differential thermal scanning analyzer (purchased from Mettler Toledo, TGA / DSC3+STAR). Using bis(2-hydroxyethyl)terephthalate (BHET, commercial grade, purity 85%) as reactant, the reactivity of the catalyst to polycondensation reaction was measured. The experimental steps are as follows. The BHET was melted at a temperature of 107˜110° C., and a catalyst with a metal content of 10 ppm, a heat stabilizer with a phosphorus content of 10 ppm, and 1% of titanium dioxide were added to form a reaction mixture. The reaction mixture was stirred until uniformly mixed. Next, the reaction mixture is cooled, and the cooled reaction mixture is ground into a powder. 10 mg of powder was placed in a 40 μL standard aluminum crucible with a lid, ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle size | aaaaa | aaaaa |
| particle size | aaaaa | aaaaa |
| particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com