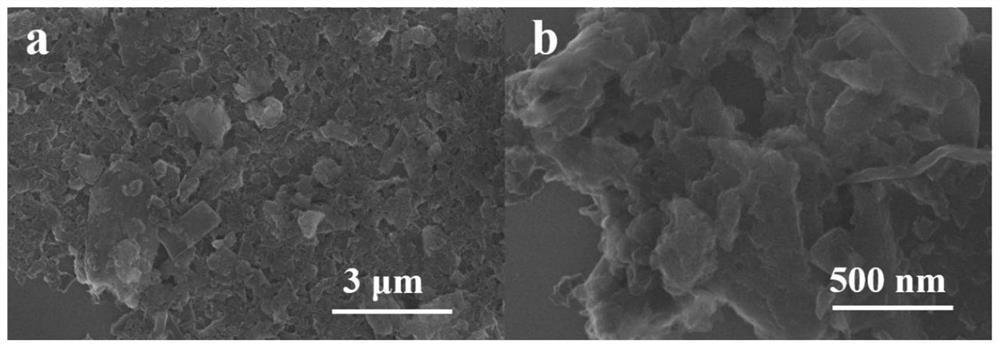
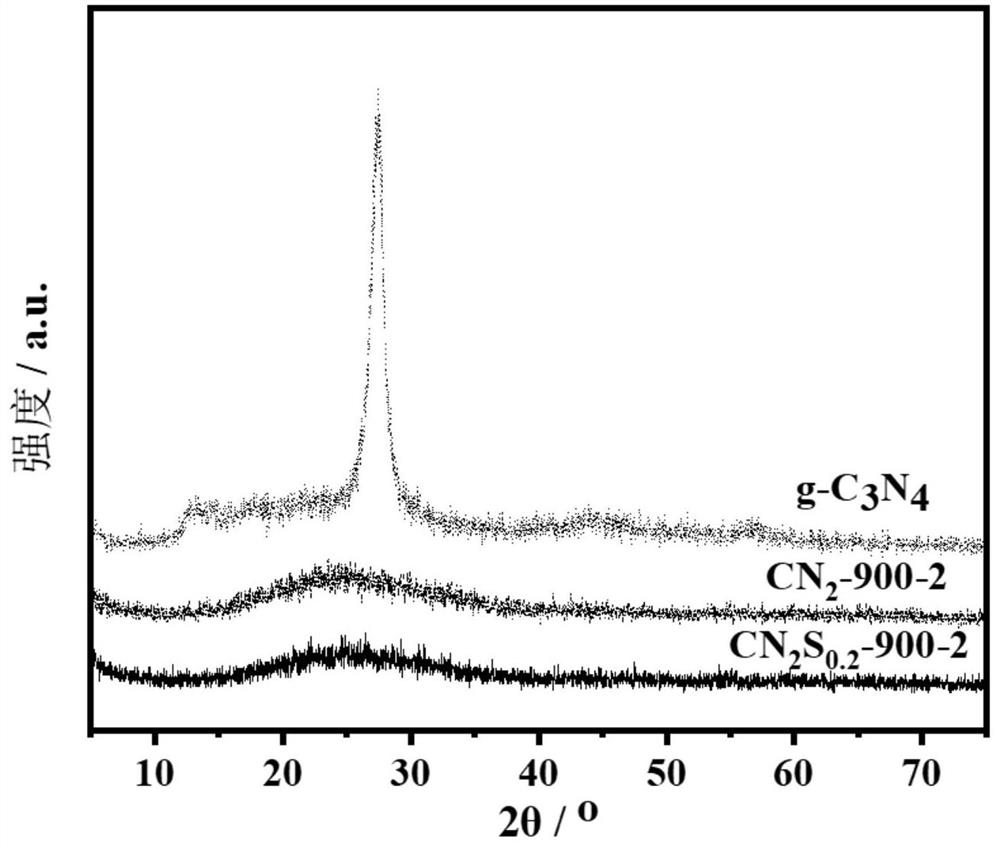
N, S co-doped metal-free CNS oxygen reduction catalyst and preparation method thereof
A catalyst and metal-free technology, applied in the direction of fuel cell half-cells and primary battery half-cells, electrical components, battery electrodes, etc., can solve the problems of complex preparation process and environmental pollution, and achieve simple preparation process and low cost. Inexpensive, good for large-scale application
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0034] Example 1: CN 1 S 0.2 -900-2(CN 1 S 0.2 1 in -900-2 means g-C in the raw material 3 N 4 The mass is 1g, 0.2 means the mass ratio of sulfur powder to sodium sulfide is 0.2, 900 means the calcination temperature is 900°C, 2 means the calcination time is 2h)
[0035] 1gg-C 3 N 4 Add to 40mL deionized water, then add 64mg sulfur powder, 280mg sodium sulfide and 200mg glucose, stir for 30min to form a uniform mixed solution A. The mixed solution A was charged into an autoclave, and reacted at 180° C. for 10 h. Then cool to room temperature naturally. Suction filtration, washing, drying in an air oven for 10 hours at 80°C, and then grinding to obtain the CNS catalyst precursor g-C 3 N 4 / CS.
[0036] will g-C 3 N 4 / CS precursor was put into the tube furnace, under N 2 It was calcined under atmosphere for 2 hours, the calcining temperature was 900°C, and the heating rate was 10°C / min. Finally got CN 1 S 0.2 -900-2 Catalyst.
Embodiment 2
[0037] Example 2: CN 2 S 0.2 -900-2(CN 2 S 0.2 2 in -900-2 means g-C in the raw material 3 N 4 The mass is 2g, 0.2 means the mass ratio of sulfur powder to sodium sulfide is 0.2, 900 means the calcination temperature is 900°C, 2 means the calcination time is 2h)
[0038] 2gg-C 3 N 4 Add to 40mL deionized water, then add 64mg sulfur powder, 280mg sodium sulfide and 200mg glucose, stir for 30min to form a uniform mixed solution A. The mixed solution A was charged into an autoclave, and reacted at 180° C. for 10 h. Then cool to room temperature naturally. Suction filtration, washing, drying in an air oven for 10 hours at 80°C, and then grinding to obtain the CNS catalyst precursor g-C 3 N 4 / CS.
[0039] will g-C 3 N 4 / CS precursor was put into the tube furnace, under N 2 It was calcined under atmosphere for 2 hours, the calcining temperature was 900°C, and the heating rate was 10°C / min. Finally got CN 2 S 0.2 -900-2 Catalyst.
Embodiment 3
[0040] Example 3: CN 3 S 0.2 -900-2(CN 3 S 0.2 3 in -900-2 means g-C in the raw material 3 N 4 The mass is 3g, 0.2 means the mass ratio of sulfur powder to sodium sulfide is 0.2, 900 means the calcination temperature is 900°C, 2 means the calcination time is 2h)
[0041] 3gg-C 3 N 4 Add to 40mL deionized water, then add 64mg sulfur powder, 280mg sodium sulfide and 200mg glucose, stir for 30min to form a uniform mixed solution A. The mixed solution A was charged into an autoclave, and reacted at 180° C. for 10 h. Then cool to room temperature naturally. Suction filtration, washing, drying in an air oven for 10 hours at 80°C, and then grinding to obtain the CNS catalyst precursor g-C 3 N 4 / CS.
[0042] will g-C 3 N 4 / CS precursor was put into the tube furnace, under N 2 It was calcined under atmosphere for 2 hours, the calcining temperature was 900°C, and the heating rate was 10°C / min. Finally got CN 3 S 0.2 -900-2 Catalyst.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Quality | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com