Redox responsive metalloporphyrin complex, preparation method thereof and preparation method of polylactic acid
A technology of metalloporphyrins and complexes, applied in chemical instruments and methods, metallocenes, organic chemistry, etc., can solve problems such as inability to control catalytic efficiency, and achieve high catalytic efficiency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
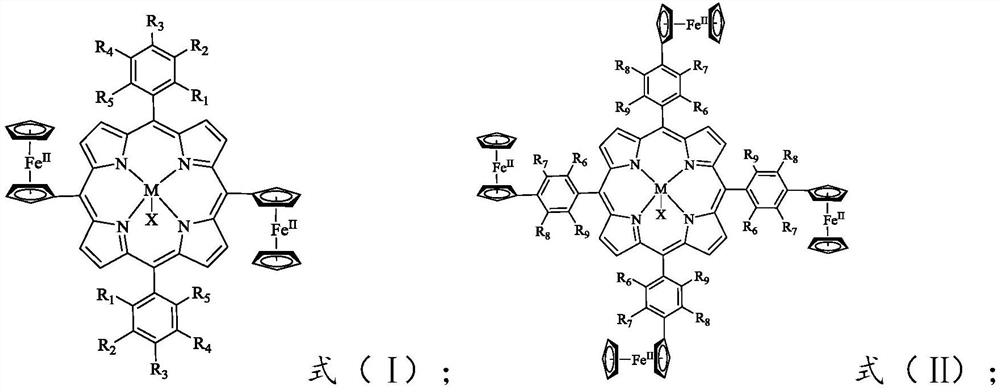
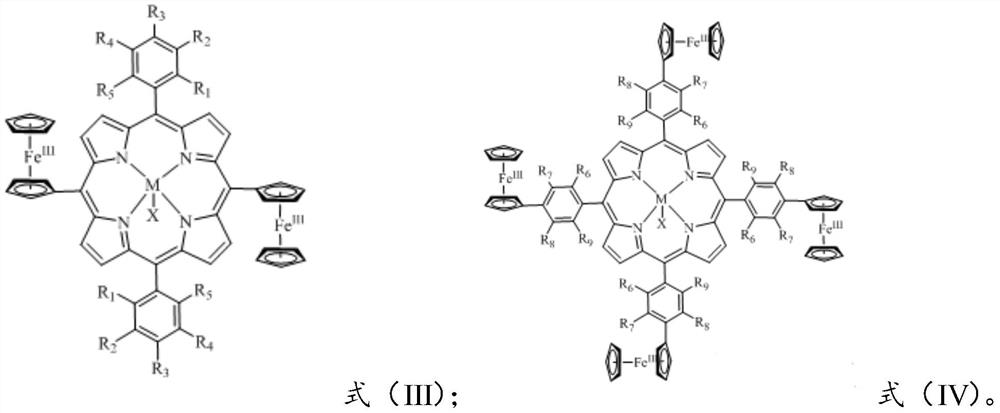
[0061] The present invention provides a preparation method of the redox-responsive metalloporphyrin complex of formula (I) or formula (II) according to any one of the above technical solutions, comprising:
[0062] Under the action of a catalyst, the compound having the structure represented by the formula (V) is subjected to a first reaction with ferrocene formaldehyde to obtain the compound having the structure represented by the formula (VI).
[0063]
[0064] The molar ratio of the compound of the structure shown in the formula (V) to the ferrocene benzaldehyde is 1:(1~1.1); the temperature of the reaction is 25°C~35°C; the time of the reaction is 1h~ 1.5h.
[0065] Reacting the compound having the structure represented by the formula (VI) with a metal salt compound in a solvent to obtain a redox-responsive metalloporphyrin complex of the formula (I);
[0066]
[0067]The molar ratio of the second compound to the metal salt compound is 1:(1~1.5); the metal in the me...
preparation example 1
[0094] Step (A-1), under anhydrous and oxygen-free reaction conditions, dissolve the first compound (20mmol) and ferrocene formaldehyde (20mmol) having the structure shown in formula (5) in 600ml of dry dichloromethane solvent , then added 1.2ml trifluoroacetic acid to the reaction solution, stirred at 25°C for 1h, then added 3.5g DDQ to the obtained reaction solution, and continued stirring at 25°C for 1h to complete the first reaction. Remove the solvent from the obtained reaction solution in vacuo to obtain a crude product, which is then purified by silica gel column chromatography (silica gel, dichloromethane / petroleum ether=1 / 1, V / V) to obtain the formula (5) The second compound of the shown structure has a yield of 18%; high-resolution electrospray mass spectrometry analysis, analysis results [C52H38Fe2N4]: 830.18, found: 830.1.
[0095]
[0096] Step (A-2), dissolving the second compound (1 mmol) of the structure shown in formula (5) obtained in step (A-1) in dry dic...
preparation example 2
[0099] Step (A-1), under anhydrous and oxygen-free reaction conditions, dissolve the first compound (20mmol) and ferrocene formaldehyde (20mmol) having the structure shown in formula (8) in 600ml of dry dichloromethane solvent , then added 1.2ml trifluoroacetic acid to the reaction solution, stirred at 25°C for 1h, then added 3.5g DDQ to the obtained reaction solution, and continued stirring at 25°C for 1h to complete the first reaction. Remove the solvent from the obtained reaction solution in vacuo to obtain a crude product, which is then purified by silica gel column chromatography (silica gel, dichloromethane / petroleum ether=1 / 1, V / V) to obtain the formula (9) The second compound with the structure shown has a yield of 21%; high-resolution electrospray mass spectrometry analysis, analysis results [C52H36Br2Fe2N4]: 986.00, found: 986.0.
[0100]
[0101] Step (A-2), dissolving the second compound (1 mmol) of the structure shown in formula (9) obtained in step (A-1) in dr...
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight distribution | aaaaa | aaaaa |
| molecular weight distribution | aaaaa | aaaaa |
| molecular weight distribution | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com