Method for activating ferricyanide and zinc ion battery prepared therefrom
A zinc-ion battery and iron ferricyanide technology, applied in battery electrodes, secondary batteries, electrolyte immobilization/gelation, etc., can solve low rate performance, specific capacity blocking PBA-cathode, rate performance capacity reduction, etc. problems, to achieve the effects of high ionic conductivity, excellent antifreeze function, and excellent cycle stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0043] This embodiment provides an antifreeze gel electrolyte, which is prepared through the following steps:
[0044] 1) Prepare 15mL 21M high-concentration bistrifluoromethanesulfonimide zinc-lithium electrolyte;
[0045] 2) Add 3 g of acrylamide to the high-concentration bistrifluoromethanesulfonimide zinc-lithium electrolyte, and stir at room temperature for 30 minutes;
[0046] 3) After adding 0.5 mg ammonium persulfate and 0.2 mg dimethylacrylamide, stir at room temperature for 30 min;
[0047] 4) Inject the product of step 3) into an ordinary glass mold, and place it in an oven at 70°C for 2 hours of polymerization;
[0048] 5) Remove the mold to obtain the final antifreeze gel electrolyte.
[0049] The present embodiment provides a kind of activation method of ferricyanide, it may further comprise the steps:
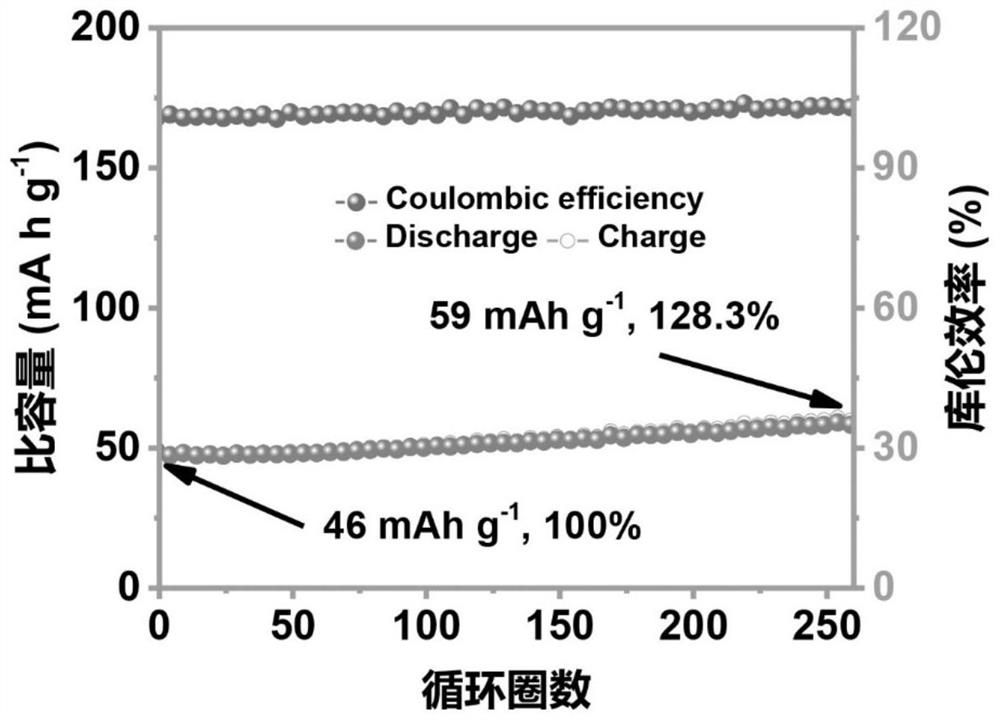
[0050] (1) The newly assembled Zn-FeHCF battery was left to stand at room temperature for 1 hour, wherein the electrolyte was the antifreeze gel electrolyte p...
Embodiment 2
[0056] The present embodiment provides a kind of activation method of ferricyanide, it may further comprise the steps:
[0057] (1) The newly assembled Zn-FeHCF battery was left to stand at room temperature for 1 hour, and the electrolyte was the antifreeze gel electrolyte prepared in Example 1;
[0058] (2) After standing still, perform a high-voltage scan on the Zn-FeHCF battery on the blue electric battery test system, and the current density is 2A g -1 , the voltage upper limit is 2.3V;
[0059] (3) After the high-voltage scan, the Zn-FeHCF battery is placed on the blue electric battery test system, and the standing time is 1s;
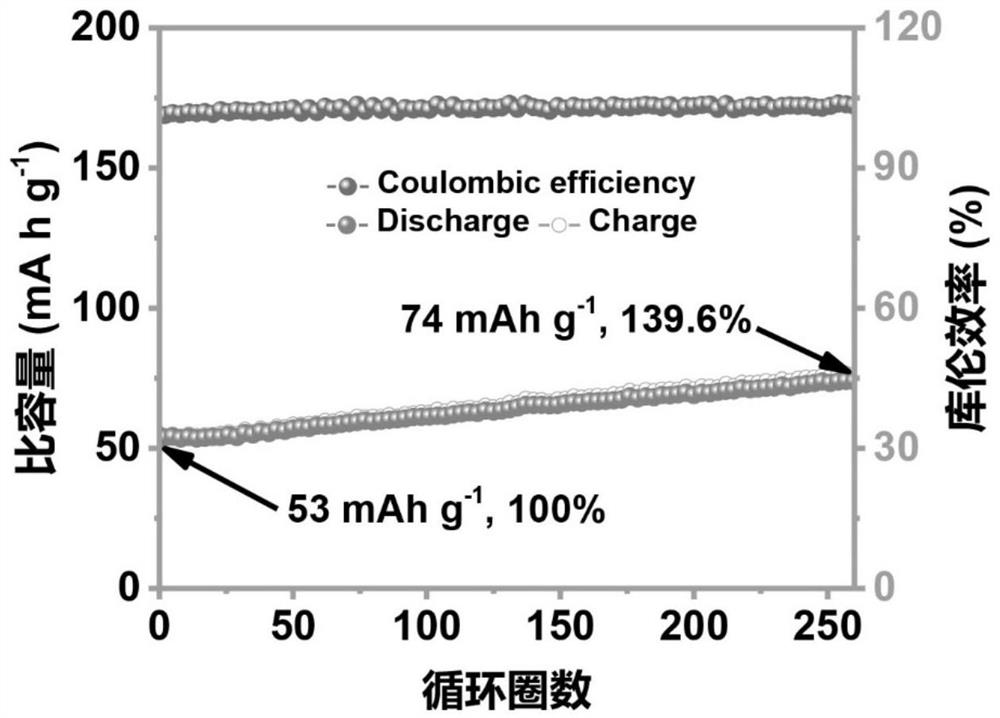
[0060] (4) After the program was left standing, the Zn-FeHCF battery was discharged on the blue electric battery test system, and the current density was 1A g -1 , the lower limit of the voltage is 0.01V; the battery completes a high-voltage scanning cycle after programmed discharge, and continues for 260 cycles to complete the activation and ob...
Embodiment 3
[0063] The present embodiment provides a kind of activation method of ferricyanide, it may further comprise the steps:
[0064] (1) The newly assembled Zn-FeHCF battery was left to stand at room temperature for 1 hour, and the electrolyte was the antifreeze gel electrolyte prepared in Example 1;
[0065] (2) After standing still, perform a high-voltage scan on the Zn-FeHCF battery on the blue electric battery test system, and the current density is 1A g -1 , the voltage upper limit is 2.3V;
[0066] (3) After the high-voltage scan, the Zn-FeHCF battery is placed on the blue electric battery test system, and the standing time is 5s;
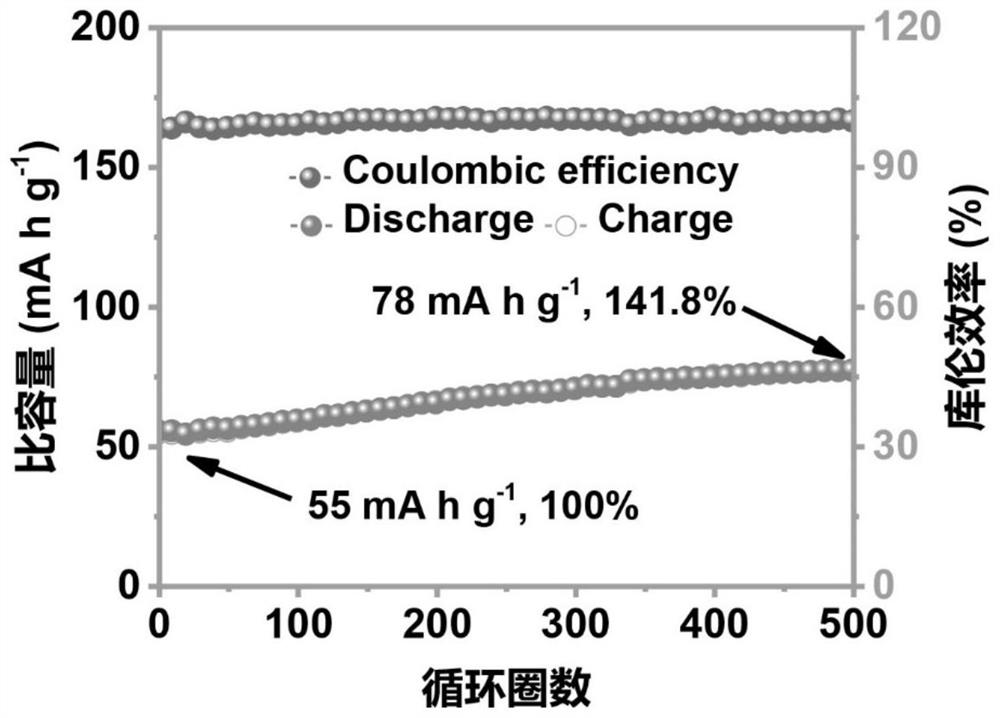
[0067] (4) After the program was left standing, the Zn-FeHCF battery was discharged on the blue electric battery test system, and the current density was 1A g -1 , the lower limit of the voltage is 0.01V; the battery completes a high-voltage scanning cycle after programmed discharge, and continues for 500 cycles to complete the activation and ob...
PUM
| Property | Measurement | Unit |
|---|---|---|
| electrical conductivity | aaaaa | aaaaa |
| current efficiency | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com