Application of Tanreqing in preparation of drugs for treating novel coronavirus pneumonia
A coronavirus and tanreqing technology, which is applied in the field of preparation of medicines for the treatment of novel coronavirus pneumonia, and achieves the effects of shortening the antipyretic time, improving cough symptoms, and shortening the coughing time.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
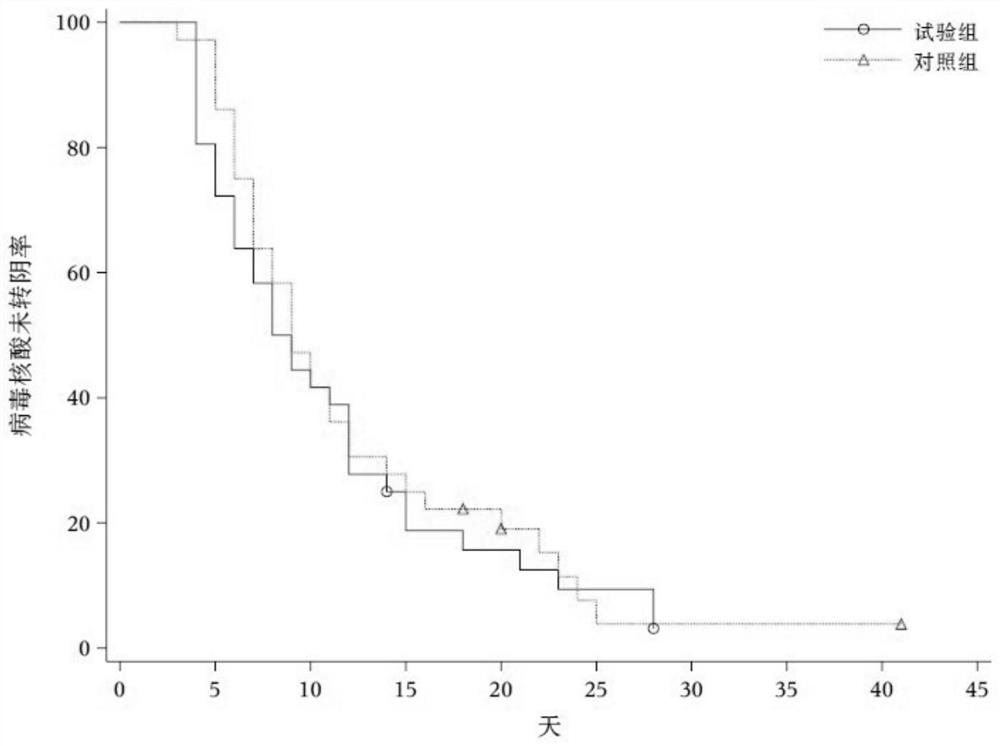
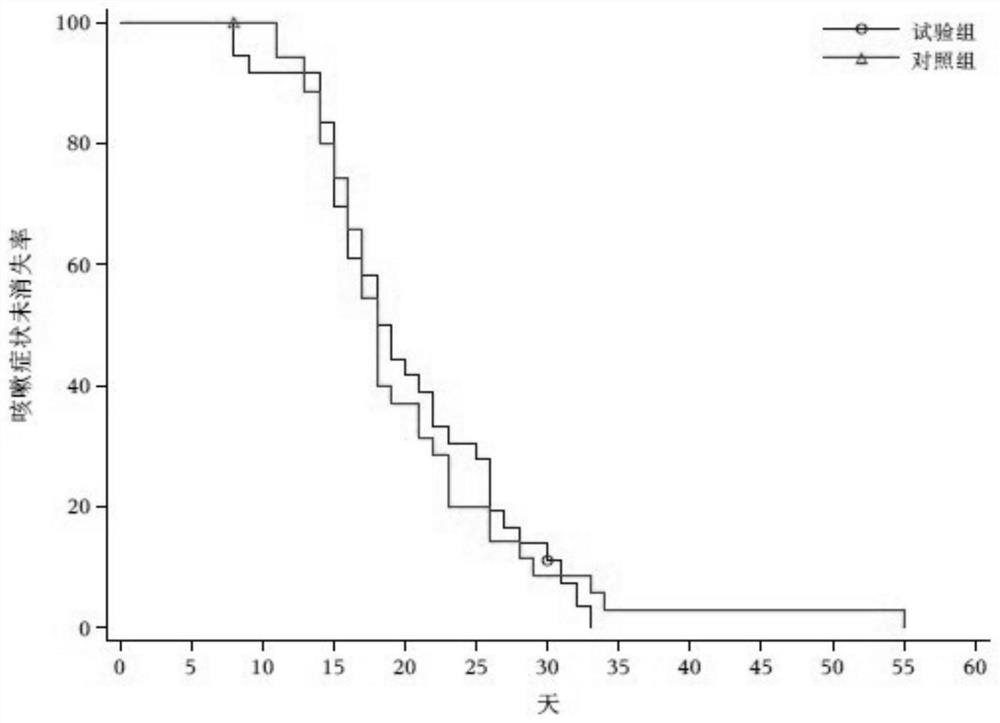
Image
Examples
Embodiment 1
[0027] Example 1 : Clinical research results of Tanreqing Capsules in the treatment of novel coronavirus pneumonia
[0028] With conventional treatment as the control, a parallel randomized, open, single-center clinical trial design is adopted:
[0029] ①Using block randomization method. Using professional statistical software, a random table of treatment (experimental group and control group) received by 72 subjects in two groups was generated, that is, the treatment allocation table corresponding to the serial number 001-072. And produce 72 random allocation letters according to the content of the random table, and the content of each letter is the group and treatment plan corresponding to each random number. After the subjects were enrolled, they opened the corresponding distribution letters in ascending order, and adopted the treatment plan shown in the letters.
[0030] ② This trial is an exploratory trial to initially evaluate the clinical effectiveness and safety of...
Embodiment 2
[0061] Example 2 : Clinical research results of Tanreqing injection in the treatment of novel coronavirus pneumonia
[0062] 1. Body temperature recovery time
[0063] Comparing the temperature recovery time between the control group and the treatment group, 3 people in the control group had no fever at the baseline level, and 4 people in the treatment group had no fever at the baseline level. The average antipyretic of the control group was 2.76 days, the median was 2 (1, 2) days, the average antipyretic of the test group was 1.68 days, the median was 3 (2, 4) days, the difference was statistically significant (P<0.01 ), suggesting that the use of Tanreqing injection in the treatment of patients with new coronary pneumonia can shorten the time for body temperature to return to normal by 1.08 days. See Table 5.
[0064] Table 5 The average time for body temperature recovery in the two groups of patients
[0065]
[0066] 2Comparison of days of hospitalization between t...
Embodiment 3
[0071] Example 3 : Mechanism of Tanreqing Capsules in the Treatment of Novel Coronavirus Pneumonia Based on Network Pharmacology
[0072] Tanreqing Capsules are made by extracting 5 medicinal materials including Scutellaria baicalensis, bear bile powder, goat horn, honeysuckle, and forsythia. On the one hand, 19 compounds of flavonoids, lignans, phenylethanol glycosides, organic acids, amino acids, and bile acids from five medicinal materials were selected as research objects, and 19 compounds were drawn using ChemBio Office 2010 The structure of scutellarin, baicalin, scutellarin-7-O-glucuronide, chrysin-7-O-glucuronide, forsythin, forsythiaside E, forsythia Esteroside D, chlorogenic acid, caffeic acid, isochlorogenic acid A, glycine, alanine, arginine, lysine, valine, tyrosine, leucine, ursodeoxycholic acid and cheese deoxycholic acid.
[0073] Through reverse docking experiments on the Swiss Target Prediction server and TCMSP database retrieval, a total of 163 related t...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com