Specific T cell receptor (TCR) directed at EGFR L858R gene mutation and application thereof
A cell receptor and specific technology, applied in the field of genetic engineering and tumor immunotherapy, can solve the problems of lack of effective TCR and the need to verify the therapeutic effect, and achieve the effect of good therapeutic effect and strong specificity.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0050] Example 1 Preparation of TCR lentivirus
[0051] The coding sequences of TCR α chain and β chain are connected by furin cleavage site, SGSG linker and F2A sequence, the α chain and β chain genes are fully synthesized, and the TCR gene is cloned into lentivirus using restriction enzymes EcoRI and BamHI In the expression vector pCDH (purchased from SBI), a recombinant plasmid is obtained, which can express the amino acid sequences of the variable regions of the TCR α chain and β chain shown in sequences 6 and 8.
[0052] The recombinant plasmid was transformed into XL-10 competent cells, spread evenly on the LB solid medium plate containing ampicillin, and after culturing at 37 °C for 12 h, a single colony was picked into the LB liquid medium containing ampicillin, and incubated at 37 ℃. Cultivate with shaking at 220rpm / min for 14-16 h, and extract plasmids.
[0053] Packaging of recombinant plasmids: Take logarithmic growth phase 293T cells (purchased from the Basic Med...
Embodiment 2
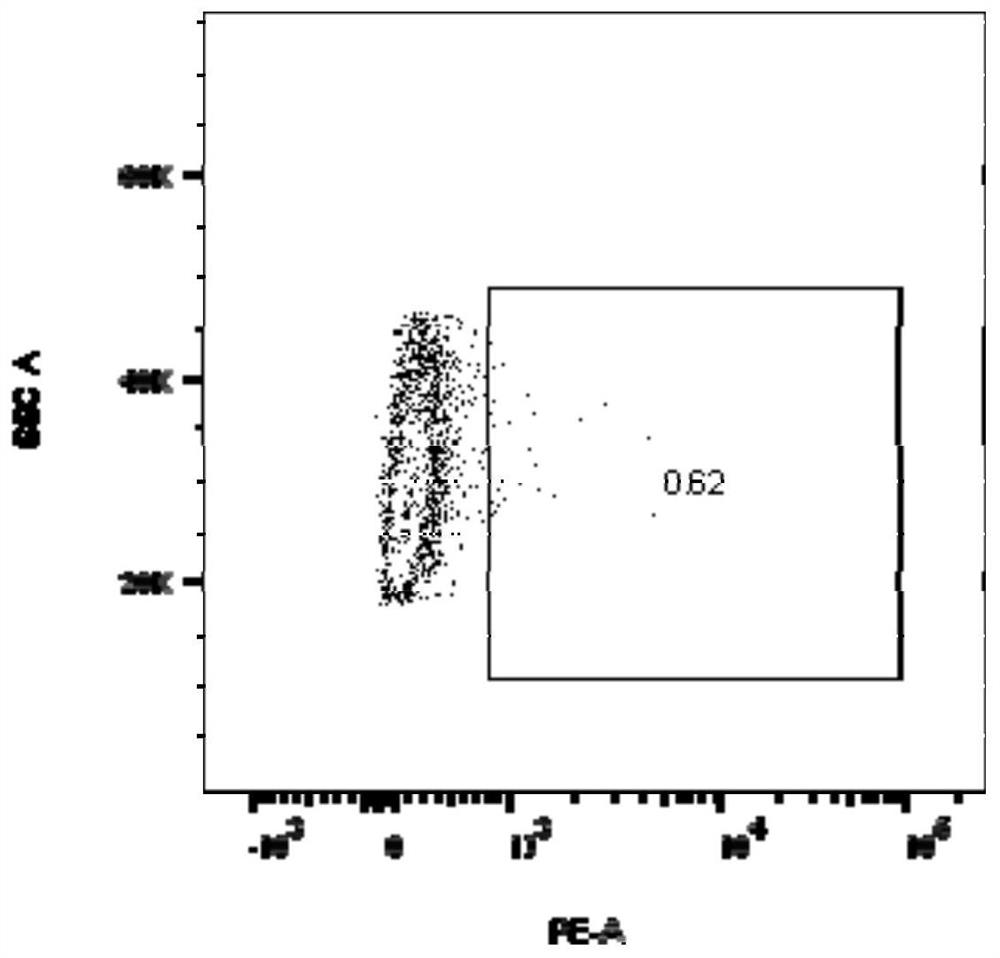
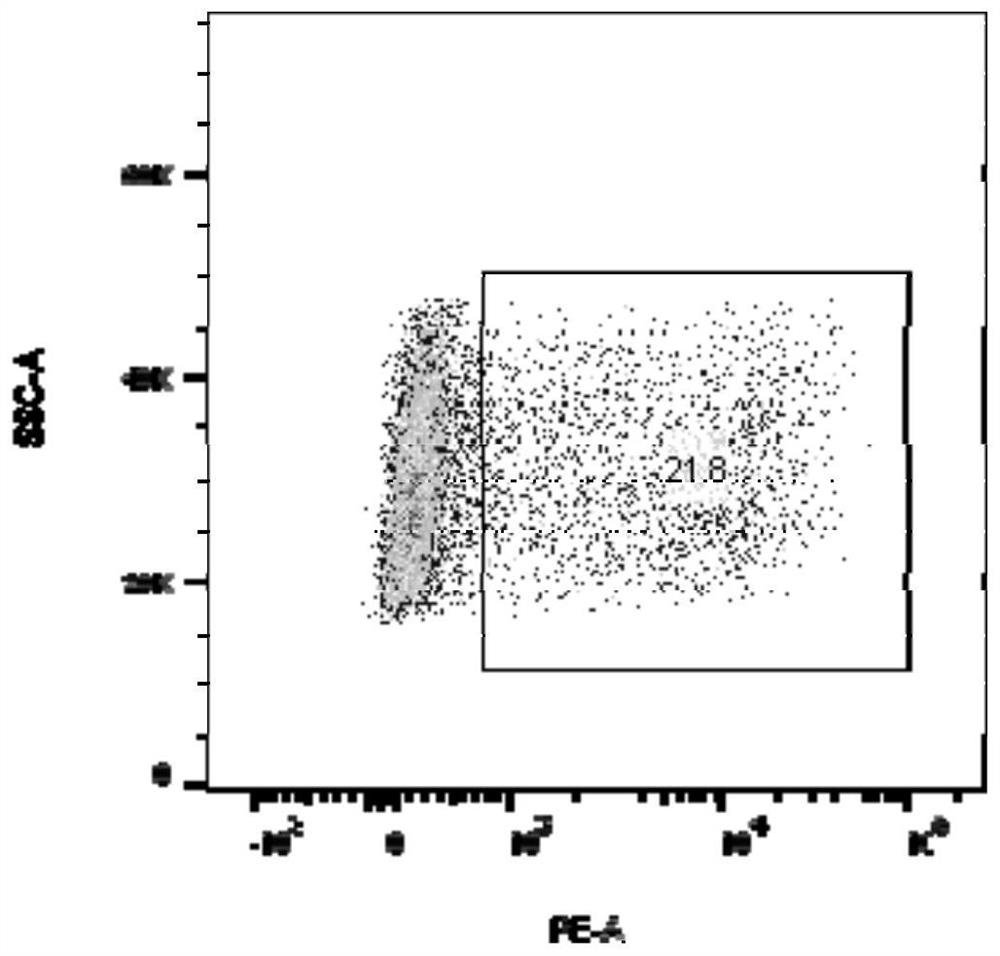
[0054] Example 2 Preparation of TCR-T cells and flow cytometry analysis of TCR expression of TCR-T cells
[0055] Peripheral blood of healthy volunteers was taken, and human peripheral blood mononuclear cells (PBMC) were obtained by using lymphocyte separation medium (Stemcell, 07861, USA). Dynabeads (Gibco, 11141D) and PBMC were mixed and incubated at room temperature for 20 min to isolate activated T cells. Add 3 mL of X-Vivo 15 medium (Lonza, DL-201) to T cells, resuspend the mixture of cells and Dynabeads (Thermo, 11141D) with a pipette, and adjust the cell density to 0.5–1 × 10 6 cells / mL to obtain a cell suspension. Place the cell suspension at 37°C, 5% CO 2 After 48 hours of continuous culture in the incubator, adjust the cell density to 1x10 6 pcs / mL. Take out the lentivirus from the -80°C ultra-low temperature freezer, quickly thaw it in a 37°C water bath, add polybrene (SantaCruz, sc-134220) to the prepared T cells to a final concentration of 6 μg / mL, add the abo...
Embodiment 3
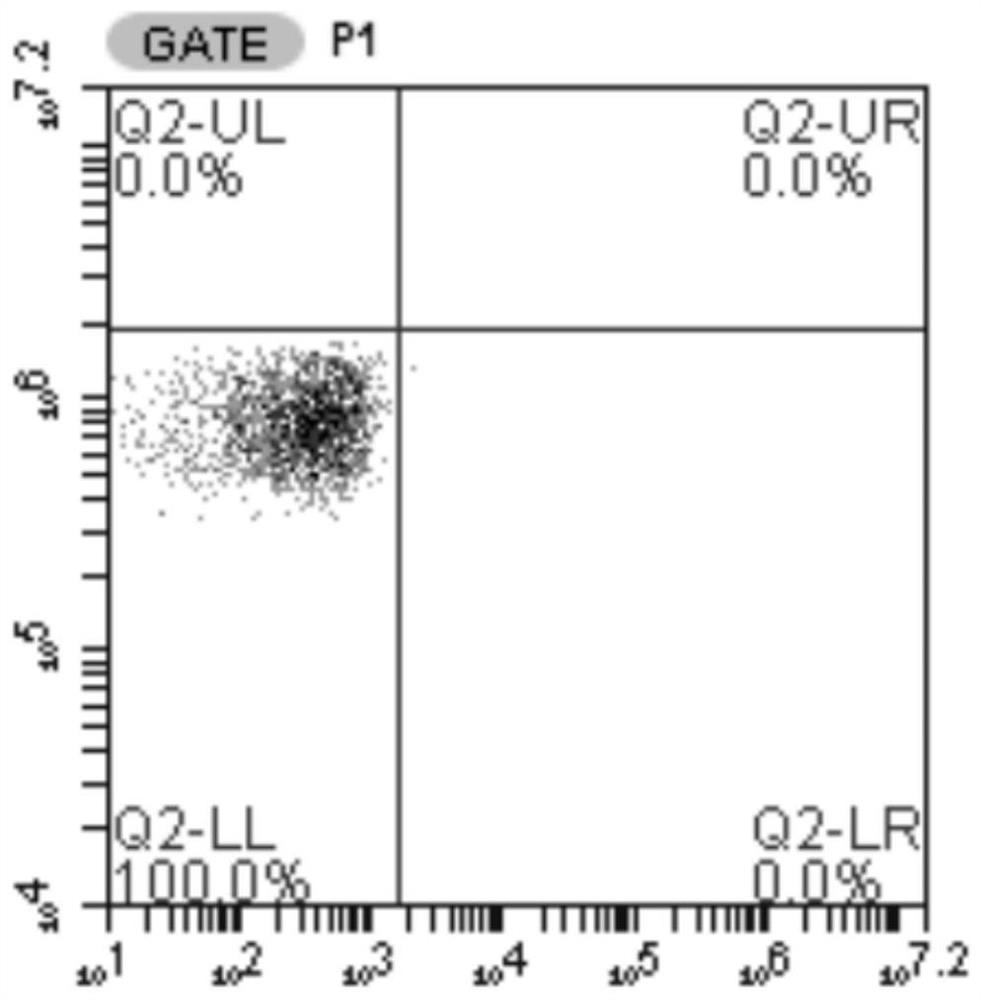
[0056] Example 3 Preparation of target cells
[0057] The lentiviral expression vector pCDH-A1101 overexpressing the HLA-A*1101 molecule was constructed, and 293T cells were transiently transfected to prepare a recombinant lentivirus. The method was the same as that in Example 1. Virus-transduced T2 cell line, according to the method of Example 2, to construct a T2 cell line expressing HLA-A*1101. After labeling with HLA molecule antibody, FACS was used to detect the expression of HLA-A*1101 on the surface of T2 cells, as shown in Figure 2. Figure 2A In the middle are T2 cells that were not transfected with HLA-A*1101, Figure 2B The middle is T2 cells transfected with HLA-A*1101, and the transfection efficiency of HLA-A*1101 was 96.6%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com