CuZn double-monatomic electrochemical catalysis CO2 reduction material and preparation method thereof
An electrochemical and atomic technology, applied in chemical instruments and methods, physical/chemical process catalysts, chemical/physical processes, etc., to solve problems such as inability to obtain catalytic performance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0045] Take 5g melamine, 5g glutamic acid, 0.5gCuCl 2 , 0.5gZnCl 2 Add it into 10 g of deionized water, stir evenly with a magnetic force, and dry the solution in an oven at 150°C. The dried powder product was placed in a tube furnace for calcination, and the temperature was raised to 800° C. at a rate of 5° C. / min under an inert gas atmosphere, and kept for 3 hours. After the heat preservation is over, it is also naturally cooled down to room temperature under an inert gas atmosphere to obtain CuZn SAs electrochemically catalyzed CO 2 Reduction of raw materials.
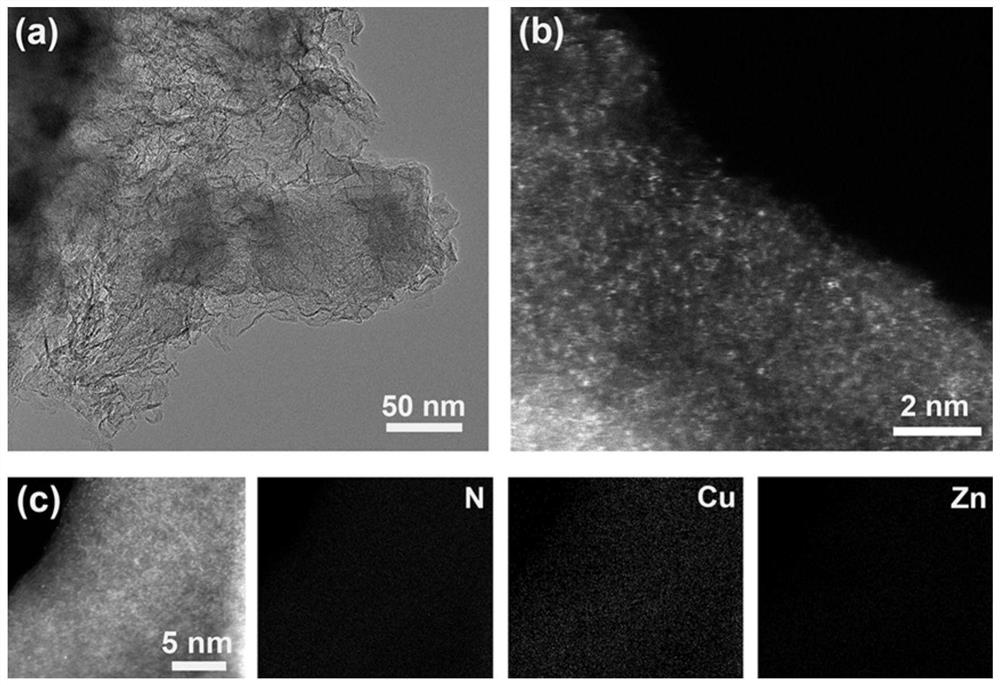
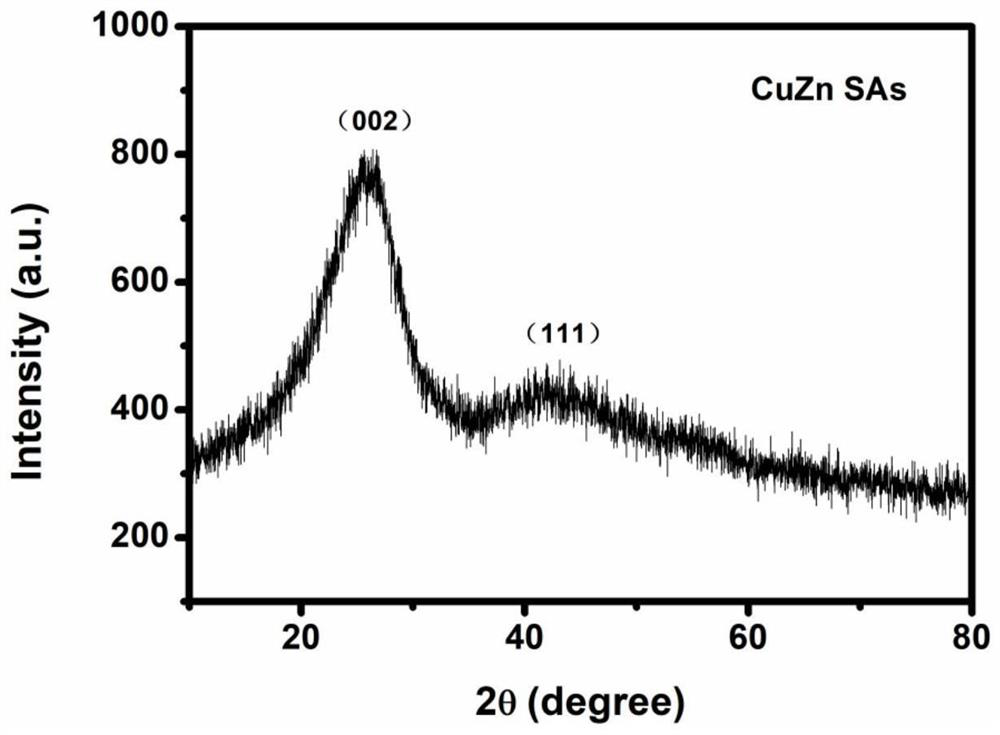
[0046] The micro-morphological characteristics of CuZn SAs electrocatalyst materials were studied by transmission electron microscopy and spherical aberration electron microscopy. figure 1 (a) is the transmission electron microscope image of CuZn SAs, composed of figure 1 (a) Two-dimensional carbon nanosheets with smooth and flat surfaces can be observed. figure 1 (b) is the spherical aberration electron micros...
Embodiment 2
[0050] Embodiment 2: the impact of metal salt dosage ratio on catalyst catalytic performance
[0051] Referring to Example 1, the CuCl 2 , ZnCl 2 The mass ratio is replaced by 1:2 (0.5gCuCl 2 , 1gZnCl 2 ) and 2:1 (1gCuCl 2 , 0.5gZnCl 2 ), other conditions remain unchanged, Cu 1 Zn 2 SAs, Cu 2 Zn 1 SAs electrochemically catalyze CO 2 Reduction of raw materials.
[0052] Test Cu according to the method in embodiment 1 1 Zn 2 SAs, Cu 2 Zn 1 SAs electrochemically catalyze CO 2 Electrochemical carbon dioxide reduction performance of reducing materials.
[0053] Cu was calculated using the relevant formula 1 Zn 2 SAs, Cu 2 Zn 1 The faradaic efficiency (FE) of SAs catalytic materials at different overpotentials is as follows: Figure 4 shown. Figure 4 (a) is Cu 1 Zn 2 SAs electrochemically catalyze CO 2 Faradaic efficiency plot of the products of reduced materials. Cu 1 Zn 2 SAs electrochemically catalyze CO 2 Products of reduced materials include...
Embodiment 3
[0054] Embodiment 3: the influence of metal salt concentration on catalyst catalytic performance
[0055] Referring to Example 1, the mass of each metal salt was replaced by 0.25g and 1g respectively, and other conditions were unchanged, and CuZn-0.25SAs and CuZn-1 SAs electrochemically catalyzed CO 2 Reduction of raw materials.
[0056] Test CuZn-0.25SAs and CuZn-1 SAs electrochemically catalyzed CO according to the method in Example 1 2 Electrochemical carbon dioxide reduction performance of reducing materials.
[0057] The faradaic efficiency values (FE) of CuZn-0.25SAs and CuZn-1 SAs catalytic materials at different overpotentials were calculated by using relevant formulas as follows: Figure 5 shown. Figure 5 (a) CuZn-0.25SAs electrochemically catalyzes CO 2 Faradaic efficiency plot of the products of reduced materials. CuZn-0.25SAs electrochemically catalyzes CO 2 Products of reduced materials include hydrogen, carbon monoxide, ethanol. And, compared with the C...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com