Hybridoma cell capable of secreting an anti-novel coronavirus N protein monoclonal antibody, onoclonal antibody and application
A monoclonal antibody, hybridoma cell line technology, applied in the field of immune detection, can solve the problem of long detection time, and achieve the effects of rapid monitoring and prevention, strong specificity and high sensitivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
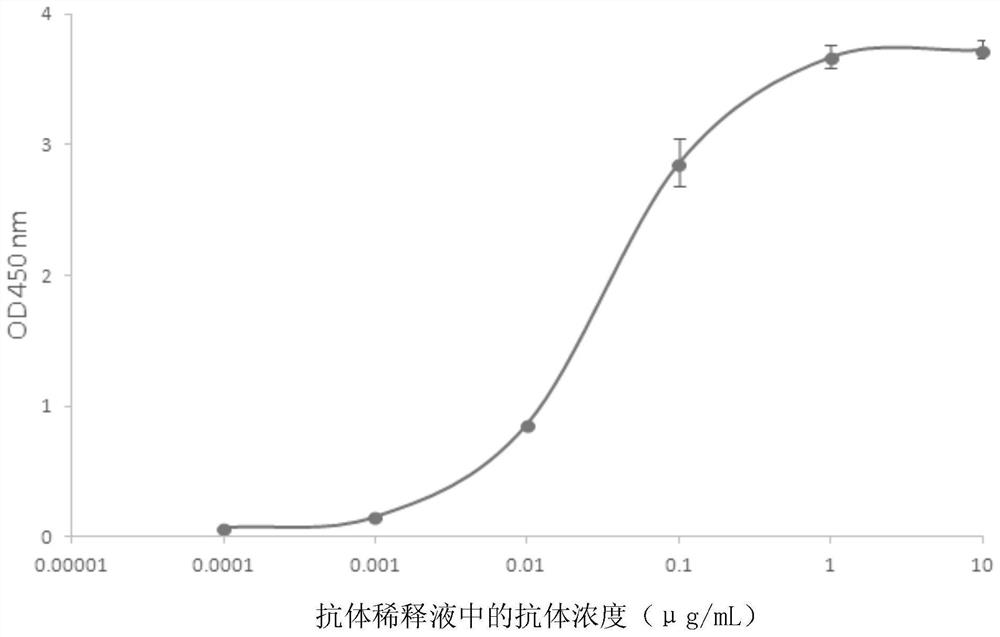
[0044] Embodiment 1, the acquisition of the hybridoma cell line that can secrete the monoclonal antibody of the N protein of anti-SARS-COV-2
[0045] The N protein of SARS-COV-2 was diluted with normal saline and mixed with an equal volume of Quick Anti-body-Mouse 3W adjuvant. Two Balb / c mice were immunized by intramuscular injection in the calf, and the immunization was boosted in the same way on the 14th day. On the 21st day, blood was collected from the tail of the mice, and the serum titer was detected by indirect ELISA after the serum was separated, and the titer reached the fusion requirement. Three days before cell fusion, a spleen boost was performed with 100 μg of protein.
[0046] Balb / c mouse spleen cells were mixed with NS1 myeloma cells in logarithmic growth phase at a ratio of 1:9, and then fused under the action of 50% PEG1500. After fusion, the cells were cultured in HAT semi-solid medium containing 10% Clone Easy medium for 8-10 days, and cell clones were sel...
Embodiment 2
[0048] Example 2, Preparation, purification and titer of monoclonal antibody N-3G3
[0049] 1. Preparation of monoclonal antibody N-3G3
[0050] 1. Incremental cultivation method
[0051] The preparation method of the cell culture medium (7.4): add fetal bovine serum to RPMI-1640 medium, and the final concentration of fetal bovine serum is 20% (mass percentage).
[0052] The hybridoma cell line N-3G3 was placed in the cell culture medium and cultured at 37°C for 3-4 days, and the cell culture supernatant was purified by Protein A affinity chromatography to obtain a monoclonal antibody (stored at -20°C).
[0053] 2. Ascites preparation
[0054] Balb / c mice were intraperitoneally injected with sterilized paraffin oil (0.5mL / only), and 10 days later, intraperitoneally injected the hybridoma cell line N-3G3 (5×10 5 cells / only), 8-10 days later, ascitic fluid was collected, purified by Protein A affinity chromatography, and then transferred to a dialysis bag for dialysis in pH7.4,...
Embodiment 3
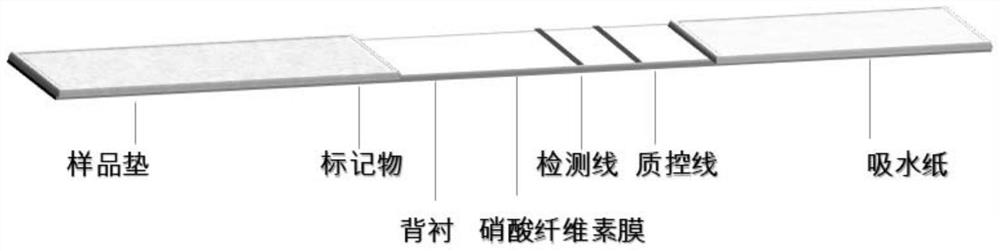
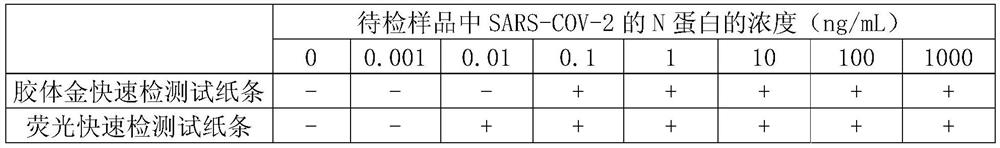
[0067] Embodiment 3, the preparation of colloidal gold rapid detection test paper
[0068] 1. Preparation of colloidal gold-labeled antibody
[0069] 1. Preparation of colloidal gold by trisodium citrate reduction method
[0070] Measure 495ml of deionized water with a measuring cylinder, pour it into a glass two-necked bottle, add 5ml of 1g / 100mL chloroauric acid aqueous solution; put a magnetic stirrer in the glass two-necked bottle, connect a spherical condenser, and use a temperature-controllable electromagnetic Heat the stirrer until boiling; add 5ml of 1g / 100mL trisodium citrate aqueous solution while stirring, the golden chloroauric acid solution turns purple within 2 minutes, continue to boil for 5 minutes, and then cool naturally, which is colloidal gold solution.
[0071] 2. Preparation of colloidal gold-labeled N-3G3 antibody
[0072] Get 20 parts by volume of colloidal gold solution, adjust the pH to 7.5 with 0.1M potassium carbonate aqueous solution, then add m...
PUM
| Property | Measurement | Unit |
|---|---|---|
| concentration | aaaaa | aaaaa |
| width | aaaaa | aaaaa |
| length | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com