Identification method and application of heparin and heparan sulfate
A technology of heparan sulfate and heparin, which is applied in the field of medical detection and achieves the effects of high sensitivity, low detection limit and quantification limit, and good repeatability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
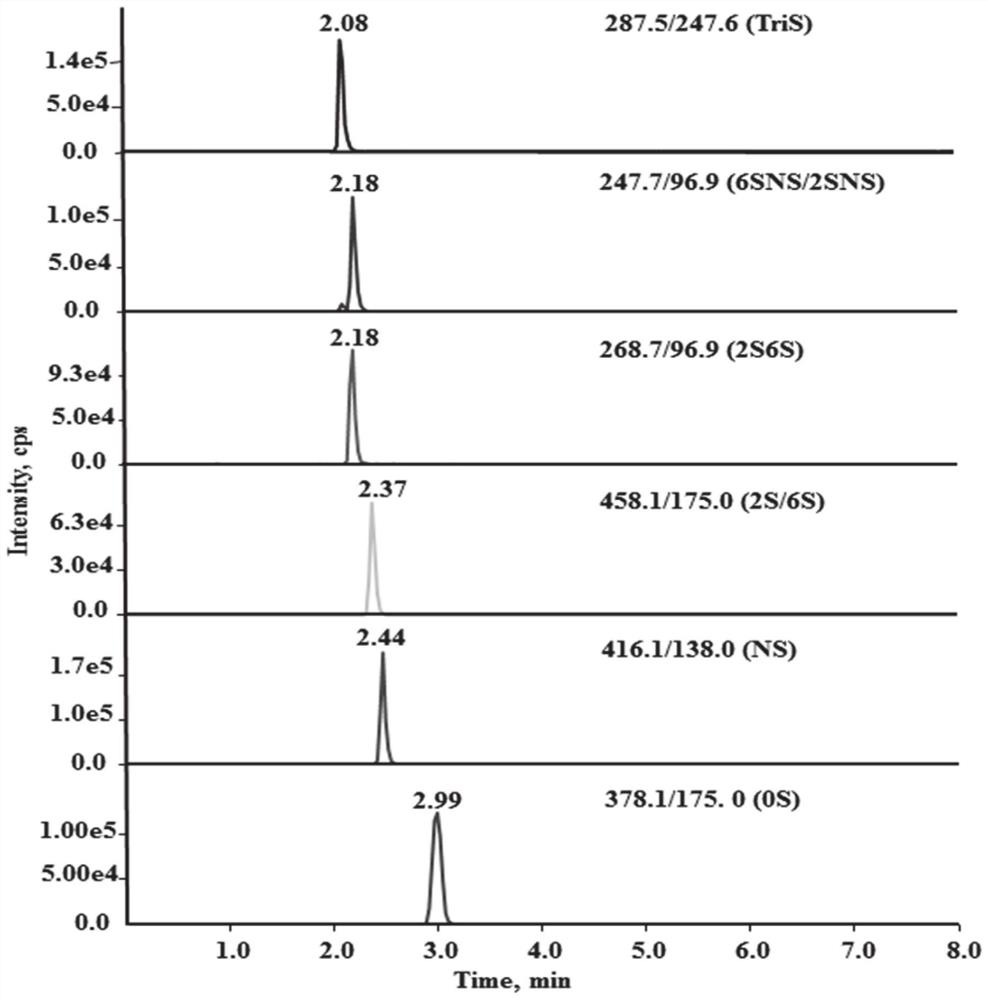
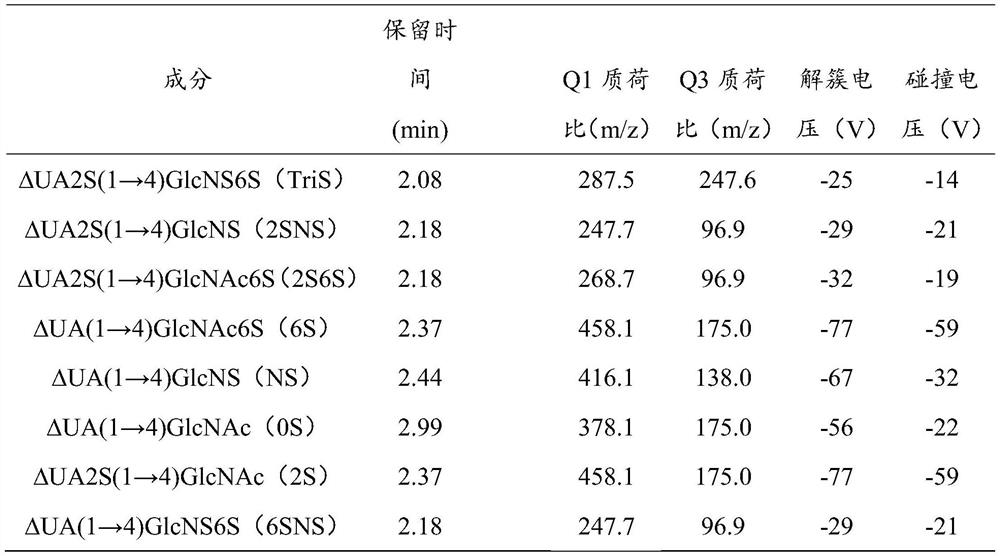
[0042] (2) Preparation of the test solution: Take 10.0 mg of the test powder, accurately weigh it, place it in a 10 mL volumetric flask, add water to dissolve and dilute to the mark, and prepare a sample stock solution with a concentration of 1.00 mg / mL. Accurately measure 100 μL of the sample stock solution, add 900 μL of enzyme reaction buffer and mix well, take 50 μL and add the mixed heparinase I, II, III (10 μL, 40 μL, 5 μL respectively), and put it in a 37°C incubator for 24 hours. After the reaction, centrifuge at 14000rpm for 10min, and take the supernatant to obtain the test solution.
[0043] (3) Determination: Accurately measure the test solution and the reference solution respectively, inject them into a high-performance liquid chromatography-mass spectrometer for separation, and adopt gradient elution, mobile phase A phase: 10mM NH 4 Ac-water, mobile phase B phase: 10mM NH 4 Ac-90% acetonitrile.
[0044] The present invention selects and optimizes to chromatogra...
Embodiment 1
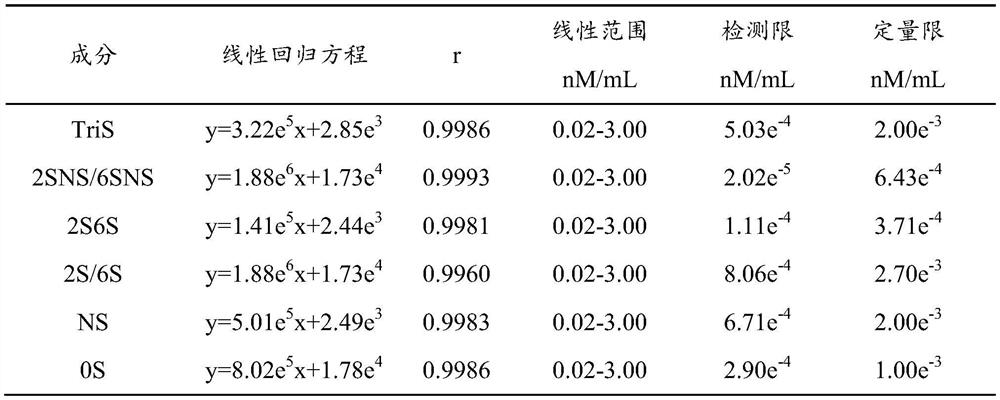
[0063] A method for liquid-mass quantitative determination of a heparin-like drug disaccharide mixed standard product, comprising the steps of:
[0064] first step:
[0065] Accurately measure an appropriate amount of the mixed standard solution, add enzyme reaction buffer and mix evenly, add the mixed heparinase I, II, III (10 μL, 40 μL, 5 μL, respectively), and react in a temperature-controlled box at 37°C for 24 hours. After the reaction, centrifuge at 14000rpm for 10min, and take the supernatant to obtain the reference solution.
[0066] Step two:
[0067] Take 10.0 mg of the test product powder, accurately weigh it, place it in a 10 mL volumetric flask, add water to dissolve and dilute to the mark, and prepare a sample stock solution with a concentration of 1.00 mg / mL. Accurately measure 100 μL of the sample stock solution, add 900 μL of enzyme reaction buffer and mix well, take 50 μL and add the mixed heparinase I, II, III (10 μL, 40 μL, 5 μL respectively), and put it ...
Embodiment 2
[0081] A kind of identification method of heparin and heparan sulfate, as follows:
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com