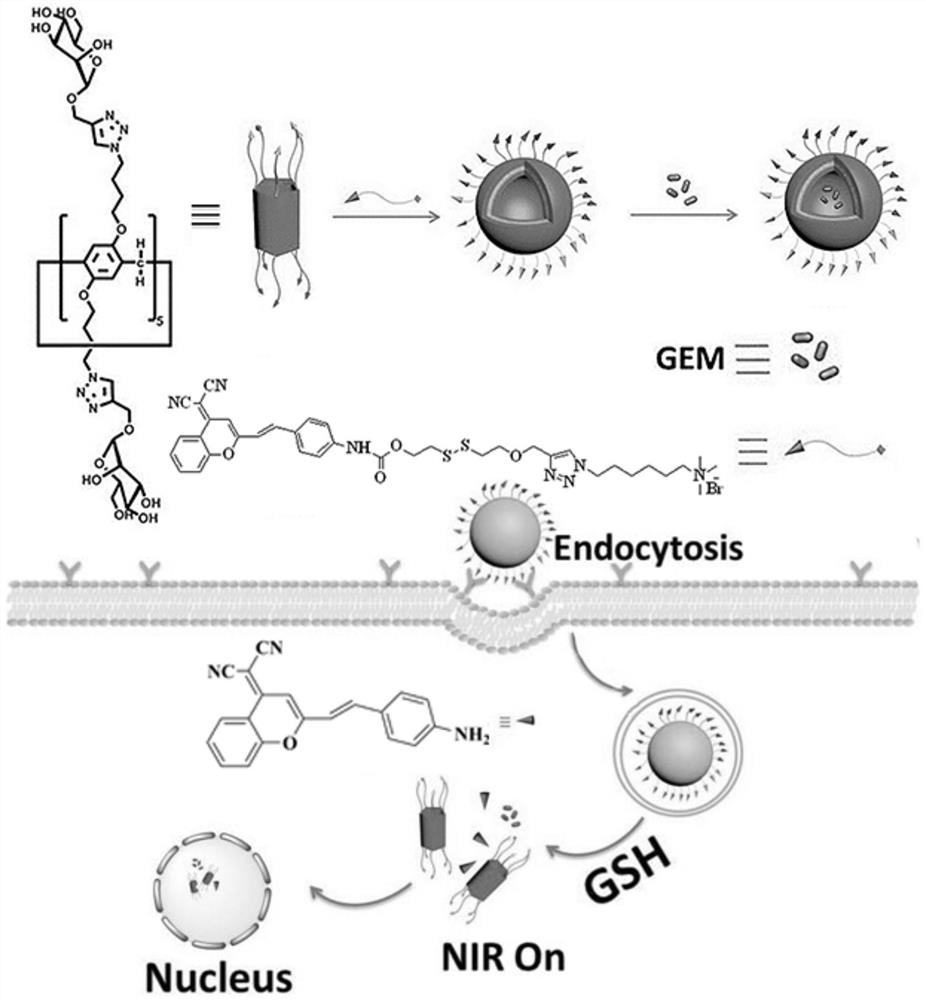
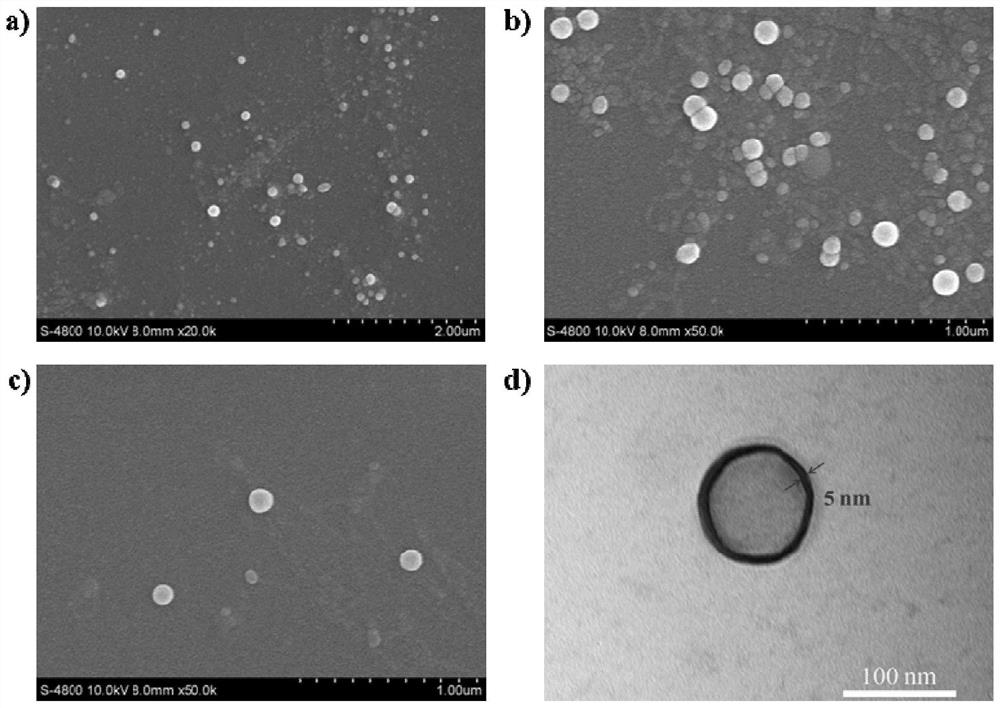
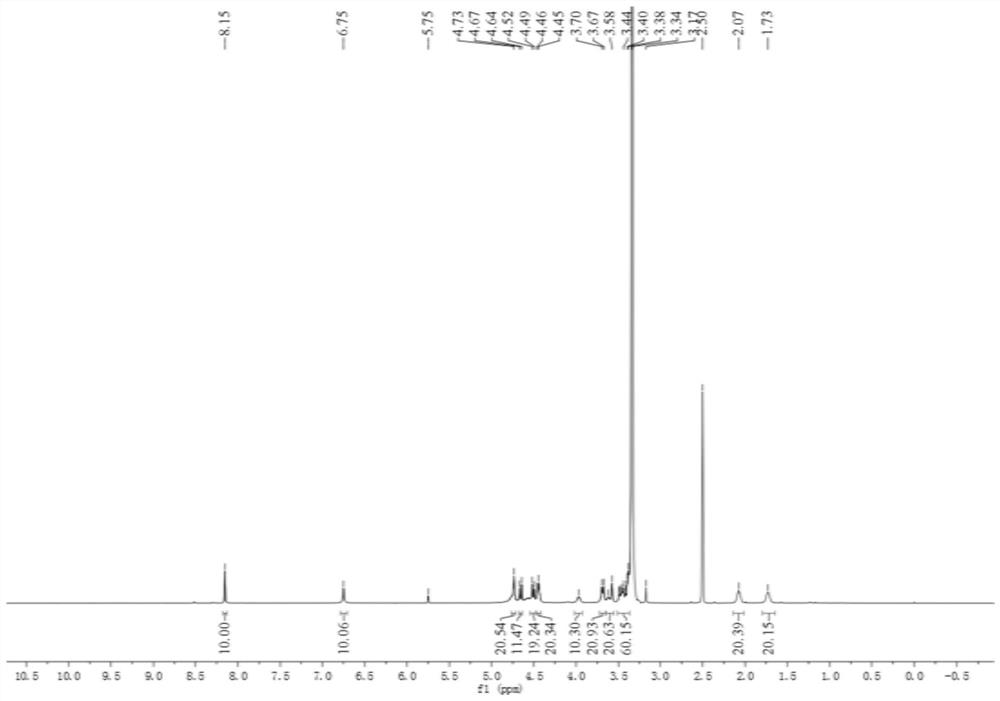
A stimuli-responsive multifunctional nanovesicle drug delivery system for targeting and fluorescent tracking
A stimuli-responsive, fluorescent tracking technology, applied in the field of nano-biomedical materials, to solve the inefficiency, achieve rapid release, and avoid the modification process.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0038] Example 1: Synthesis of Compound 1
[0039] The synthetic route of compound 1 is as follows:
[0040]
[0041] Accurately weigh hydroquinone (5.5g, 50mmol) and 1,4-dibromobutane (41.4g, 300mmol) in a round-bottomed flask, add potassium carbonate (43.2g, 200mmol), under nitrogen protection, use Acetone (300 mL) was stirred to dissolve, and then heated to reflux for 48 h. After the reaction was completed, it was poured into ice water to quench, the residue was dissolved in dichloromethane after filtration, and washed with deionized water, the organic phases were combined, and anhydrous MgSO 4 After drying and filtration, the filtrate was distilled under reduced pressure to obtain the crude product, and finally column chromatography separation (eluent: petroleum ether / dichloromethane=1:1, v / v) gave compound 1 as a white solid.
Embodiment 2
[0042] Example 2: Synthesis of Compound 2
[0043] The synthetic route of compound 2 is as follows:
[0044]
[0045] Accurately weigh compound 1 (641.7 mg, 1.69 mmol) and add it to a 100 mL round-bottomed flask, then add paraformaldehyde (65.8 mg, 0.73 mmol) and 1,2-dichloroethane (20 mL), stir for 10 min, and add Boron trifluoride ether (0.271 g, 0.2 mmol) was added to quench with ice water after the reaction for 2 h. After separation and washing with water, the organic phases were combined, dried over anhydrous sodium sulfate, and filtered. The filtrate was distilled under reduced pressure to remove the solvent. Finally, column chromatography separation (eluent: petroleum ether / dichloromethane=1:1, v / v) gave compound 2 as a white solid.
Embodiment 3
[0046] Example 3: Synthesis of Compound 3
[0047] The synthetic route of compound 3 is as follows:
[0048]
[0049] Compound 2 (101 mg, 0.05 mmol) was weighed and dissolved in N,N-dimethylformamide (14 mL), and NaN was added. 3 (36 mg, 0.55 mmol), stirred and heated to 80 °C for 12 h. After the reaction was completed, it was cooled to room temperature, 30 mL of dichloromethane was added, washed with water (3×15 mL), then washed with saturated brine (3×15 mL), and finally dried with anhydrous sodium sulfate, filtered, and then distilled under reduced pressure to remove the solvent, Finally, column chromatography was used to obtain compound 3 as a white solid.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com