A kind of preparation method of rabeprazole chloride and intermediate thereof
A technology of pyridine nitrogen oxide and methoxypropoxy, applied in the field of drug synthesis, can solve the problems of long time, high reaction temperature, long cycle and the like, and achieve the effects of easy operation and mild reaction conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 13
[0047] Example 13 Preparation of methyl-2-hydroxymethyl-4-(3-methoxypropoxy)pyridine
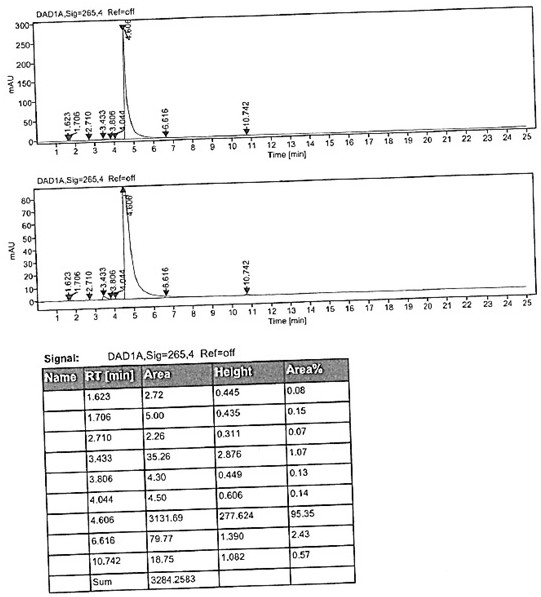
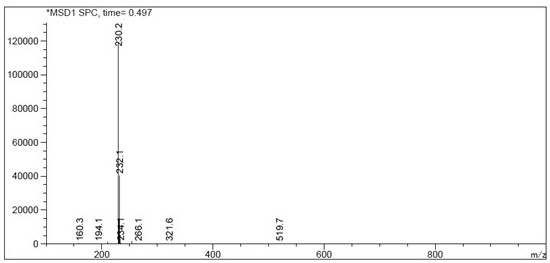
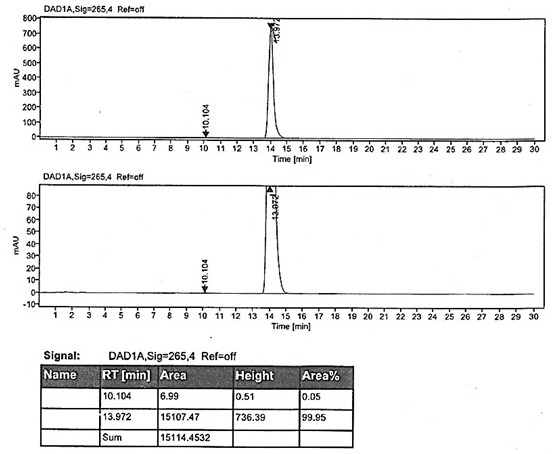
[0048]Add 50g of 2,3-dimethyl-4-(3-methoxypropoxy)pyridine nitrogen oxide, 48.5g of acetic anhydride and 4g of p-toluenesulfonic acid into the reaction flask, stir evenly, then heat up and control the temperature to 50±5 After 3 hours of reaction at °C, TLC showed that the reaction of the raw materials was complete (HPLC detected the reaction liquid, the purity of the target product was 93.6%), and the excess acetic anhydride was concentrated under negative pressure. Add 10% aqueous sodium hydroxide solution, adjust the pH to 14, and then heat up to 80 ° C for 1 hour. After TLC confirms that the reaction is complete, it is lowered to room temperature. After extraction with toluene, it is concentrated to dryness to obtain the compound shown in the title. The molar yield is 90.1 %, purity 94.71%.
Embodiment 23
[0049] Example 23 Preparation of methyl-2-hydroxymethyl-4-(3-methoxypropoxy)pyridine
[0050] Add 50g of 2,3-dimethyl-4-(3-methoxypropoxy)pyridine nitrogen oxide, 48.5g of acetic anhydride and 8g of p-toluenesulfonic acid into the reaction flask, stir evenly, then heat up, and control the temperature to 60±5 After 3 hours of reaction at ℃, TLC showed that the reaction of the raw materials was complete (HPLC detected the reaction liquid, the purity of the target product was 95.1%), and the excess acetic anhydride was concentrated under negative pressure. Add 10% aqueous sodium hydroxide solution, adjust the pH to 14, then heat up to 80 ° C for 1 hour, after confirming that the reaction is complete by TLC, it is lowered to room temperature, extracted with toluene and concentrated to dryness to obtain the compound shown in the title, with a molar yield of 91.6 %, purity 95.35%.
Embodiment 33
[0051] Example 3. Preparation of 3-methyl-2-hydroxymethyl-4-(3-methoxypropoxy)pyridine
[0052] Add 50g of 2,3-dimethyl-4-(3-methoxypropoxy)pyridine nitrogen oxide, 97g of acetic anhydride and 4g of p-toluenesulfonic acid into the reaction flask, stir evenly, then heat up, and control the temperature to 50±5℃ After 3 hours of reaction, TLC showed that the reaction of the raw materials was complete (HPLC detected the reaction solution, the purity of the target product was 94.9%), and the excess acetic anhydride was concentrated under negative pressure. Add 10% aqueous sodium hydroxide solution, adjust the pH to 14, and then heat up to 80 ° C for 1 hour. After TLC confirms that the reaction is complete, it is lowered to room temperature. After extraction with toluene, it is concentrated to dryness to obtain the compound shown in the title. The molar yield is 92.1 %, purity 95.52%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com