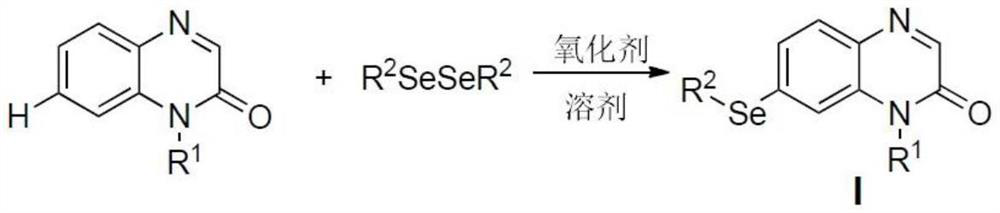
Method for regioselectively synthesizing 7-arylseleno quinoxalinone derivative
A technology of quinoxalinone and synthetic method, which is applied in the field of synthesis of 7-arylselenoquinoxalinone derivatives, can solve the problems of narrow application range of substrates, etc., achieve easy preparation of raw materials, reduce waste of resources, reduce Effects of Environmental Pollution
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0033] Example 1.R 1 =-Me, R 2 When =-Ph, the preparation of 1-methyl-7-phenylselenoquinoxalin-2 (1H)-one derivatives
[0034] Add 1-methylquinoxalin-2(1H)-one (0.2mmol, 32.0mg) and diphenyldiselenide (0.2mmol, 62.8mg) in a 25mL round bottom flask, then add 70% peroxygen An aqueous solution of tert-butanol (0.5mmol, 65.0mg), and finally 2mL of 1,2-dichloroethane was added as a solvent. React at 80°C for 5 hours; after the reaction, remove the solvent under reduced pressure, add 10 mL of ethyl acetate to the residue, wash twice with 20 mL of saturated brine; wash the organic layer with anhydrous Na 2 SO 4 After drying and concentration under reduced pressure, it was separated and purified by column chromatography (eluent: ethyl acetate / petroleum ether=1 / 5) to obtain 0.054 g of a colorless solid with a yield of 85.0%.
[0035]
[0036]1 H NMR (400MHz, CDCl 3 )δ:8.26(s,1H),7.95(d,J H-H =1.9Hz,1H),7.65(dd,J H-H =8.6Hz,J H-H =1.9Hz,1H),7.50-7.48(m,2H),7.30-7.27(m,3H),7.2...
Embodiment 2
[0037] Example 2.R 1 =-CH 2 CH 2 CH 3 , R 2 =-Ph, the preparation of 1-n-propyl-7-phenylselenoquinoxalin-2 (1H)-one derivatives
[0038] Add 1-propylquinoxaline-2(1H)-one (0.2mmol, 37.6mg) and diphenyldiselenide (0.2mmol, 62.8mg) into a 25mL round bottom flask, then add di-tert-butoxy Base peroxide (0.5mmol, 73.0mg), and finally add 2mL DMF as solvent. React at 90°C for 6 hours; after the reaction, remove the solvent under reduced pressure, add 10 mL of ethyl acetate to the residue, wash twice with 20 mL of saturated brine; wash the organic layer with anhydrous Na 2 SO 4 After drying and concentration under reduced pressure, it was separated and purified by column chromatography (eluent: ethyl acetate / petroleum ether=1 / 6) to obtain 0.060 g of a colorless solid with a yield of 87.0%.
[0039]
[0040] 1 H NMR (400MHz, CDCl 3 )δ:8.23(s,1H),7.94(d,J H-H =1.9Hz,1H),7.61(dd,J H-H =8.7Hz,J H-H =1.9Hz,1H),7.49-7.46(m,2H),7.26-7.24(m,3H),7.21(d,J H-H =8.7Hz, 1H), 4.13(...
Embodiment 3
[0041] Example 3.R 1 =-CH 2 COOC 2 h 5 , R 2 When =-Ph, the preparation of 1-ethoxyethyl-7-phenylselenoquinoxalin-2 (1H)-one derivatives
[0042] In a 25mL round bottom flask, add 1-ethoxyethylquinoxalin-2(1H)-one (0.2mmol, 46.4 mg) and diphenyl diselenide (0.2mmol, 62.8mg), then add Selectfluor Fluorine reagent (0.5mmol, 177.0mg), finally add 1mL MeCN and 1mL H 2 O is a mixed solvent. React at 80°C for 2 h; after the reaction, remove the solvent under reduced pressure, add 10 mL of ethyl acetate to the residue, wash twice with 20 mL of saturated brine; wash the organic layer with anhydrous Na 2 SO 4 After drying and concentration under reduced pressure, it was separated and purified by column chromatography (eluent: ethyl acetate / petroleum ether=1 / 3) to obtain 0.070 g of a colorless solid with a yield of 90.0%.
[0043]
[0044] 1 H NMR (400MHz, CDCl 3 )δ:8.31(s,1H),7.97(d,J H-H =1.9Hz,1H),7.61(dd,J H-H =8.6Hz,J H-H =1.9Hz,1H),7.52-7.50(m,2H),7.31-7.29(m,3H),6...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com