A method for preparing polypeptide drug microspheres based on subliquid jet spray technology
A technology of polypeptides and airflow nozzles, which is used in pharmaceutical formulations, microcapsules, nanocapsules, etc., can solve the problems of low spheroidization rate of microspheres, easy aggregation, and difficulty in realization of microspheres, and achieves high yield and drug production. Release uniform and long-lasting effects with low equipment cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment approach
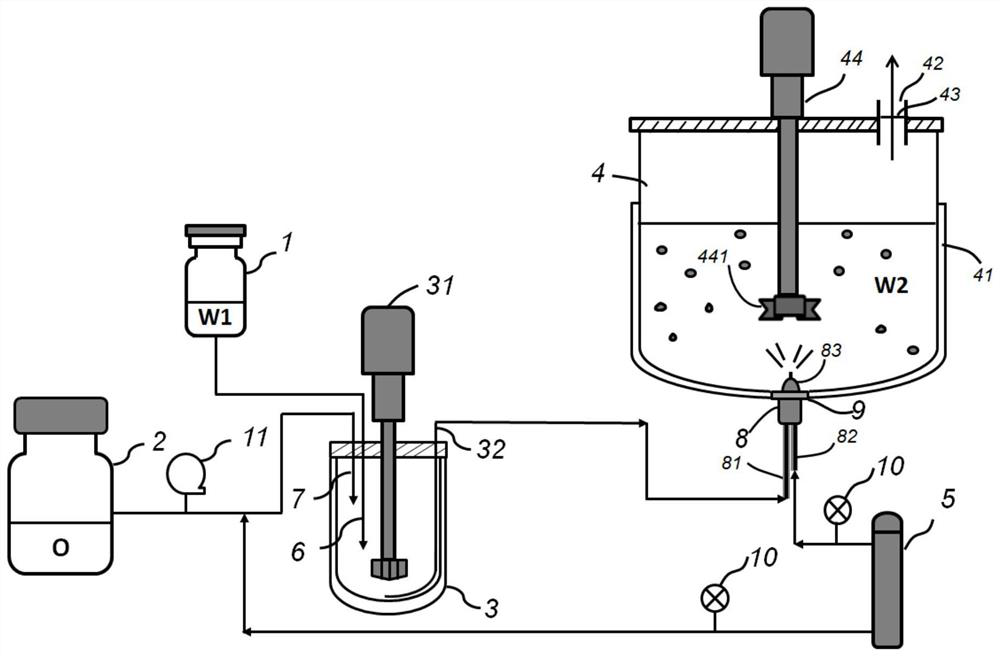
[0040] A method for preparing polypeptide drug microspheres based on submerged airflow spray technology, comprising the following steps:
[0041](1) Dissolving the polypeptide water-soluble drug in water to obtain an inner aqueous drug solution with a concentration of 40-55wt%; dissolving the carrier material in an organic solvent to obtain a phase polymer solution with a concentration of 25-40wt% oil; The water-soluble drug is selected from one of leuprolide acetate, triptorelin acetate, goserelin acetate, octreotide, exenatide, lanreotide and pasireotide; the carrier material is PLA or PLGA; the organic solvent is selected from one of dichloromethane, ethyl acetate and chloroform.
[0042] (2) adding the oil phase polymer solution to the inner water phase drug solution, the volume ratio of the inner water phase drug solution and the oil phase polymer solution is controlled to be 1: 11-22, and ultrasonic crushing or shear dispersion forms Colostrum, the colostrum particle si...
Embodiment 1
[0050] (1) dissolving leuprolide acetate in water to obtain an inner water phase with a drug concentration of 50%; dissolving PLGA (7525, Mw13000Da) at the carboxyl end in dichloromethane to obtain an oil with a polymer concentration of 33.3% Mutually;
[0051] (2) adding the above-mentioned oil phase to the inner water phase (volume ratio 12.5:1), controlling the colostrum shearing temperature to be 15°C, and using a high-speed shearing machine at 12000rpm to shear for 10min to form a primary emulsion, and the primary emulsion viscosity is 1541cp (20.83°C);
[0052] (3) setting the airflow velocity to be 130L / min, the above-mentioned initial emulsion is supplied into the liquid inlet of the airflow nozzle at a constant speed with a flow rate of 10mL / min, after the compressed air flow is broken, the bottom of the drying tank in the liquid is sprayed into 15 ℃ from bottom to top In a 0.1% concentration polyvinyl alcohol aqueous solution, under stirring at 300 rpm, a well-dispe...
Embodiment 2
[0059] (1) dissolving leuprolide acetate in water to obtain an inner water phase with a drug concentration of 50%; PLGA (7525, Mw 13000Da) at the carboxyl end is dissolved in dichloromethane to obtain a polymer concentration of 37.5%. oil phase;
[0060] (2) adding the above-mentioned oil phase to the inner water phase (volume ratio 12.5:1), controlling the colostrum shearing temperature to be 17°C, and shearing for 10 min under the condition of a high-speed shearing machine at 12000 rpm to form a primary emulsion, and the initial emulsion viscosity is 2499cp (18.94°C);
[0061] (3) setting the airflow flow rate to be 130L / min, the above-mentioned initial emulsion is supplied into the liquid inlet of the airflow nozzle at a constant speed with a flow rate of 10mL / min, after the compressed air flow is broken, the bottom of the drying tank in the liquid is sprayed into 17 ℃ from bottom to top In a 0.1% concentration polyvinyl alcohol aqueous solution, under stirring at 300 rpm,...
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
| particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com