Conjugate of anti-human DLL4 humanized antibody and dolastatin derivative MMAE as well as preparation method and application of conjugate
A technology of humanized antibody and dolastatin, which is applied in the direction of non-active ingredient medical preparations, drug combinations, anti-tumor drugs, etc., can solve the problem of wrong intra-chain or inter-chain disulfide bonds, coupling drug linker molecule inconsistency, etc. Stability, difficult to dissociate and other issues
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0064] Using molecular biology technology to prepare the engineered anti-human DLL4 humanized antibody THL4 (including the anti-human DLL4 humanized antibody H3L2 (naked antibody) preparation method obtained by site-directed mutagenesis of the anti-human DLL4 humanized antibody H3L2, as in the reference literature [ 1].): (1) According to the nucleic acid sequences of the light chain and heavy chain of the humanized antibody H3L2, determine the corresponding antibody light chain 207 (valine) and heavy chain 121 (lysine), and design primers (8 items in total), mutation into the nucleotide corresponding to cysteine by Overlap-PCR; (2) TA cloning, transforming the target fragment into E. coli DH5α host strain, and picking a single clone for DNA sequencing , The sequence of the correct sequence was digested and linked to the two expression plasmids pMH3 (neomycin resistance) and pCA-puro (puromycin resistance). After DNA sequencing was correct, 4 engineering plasmids were obtained...
Embodiment 2
[0067] The coupling between MMAE and the engineered anti-human DLL4 humanized antibody THL4 was detected by HPLC.
[0068] Agilent 1200HPLC analyzes the coupling of antibody-drug conjugate HLvM4. The sample detection conditions are as follows: (1) Mobile phase A: 20mmol / LPBS(pH 7.0)+1.5mol / L ammonium sulfate; (2) Mobile phase B: 20mmol / LPBS(pH7.0) / isopropanol=7.5 / 2.5 (3) Elution gradient: 10-100% B; (4) Elution time: 20 min; (5) Flow rate: 0.60 mL / min; (6) Injection volume: 10 uL; (7) Detection wavelength: 280 nm. According to the number of peaks and the area of each peak, the antibody-drug coupling ratio (DAR) is calculated proportionally.
[0069] See the results of the experiment figure 2 Compared with H3L2, which has the main peak at 9.min, HLvM4 also peaks at 11min, 13.5min, 17min and 19min, respectively. The number of MMAE small molecules corresponding to the coupling is 2, 4, 6 and 8 and the corresponding peak area ratio
Embodiment 3
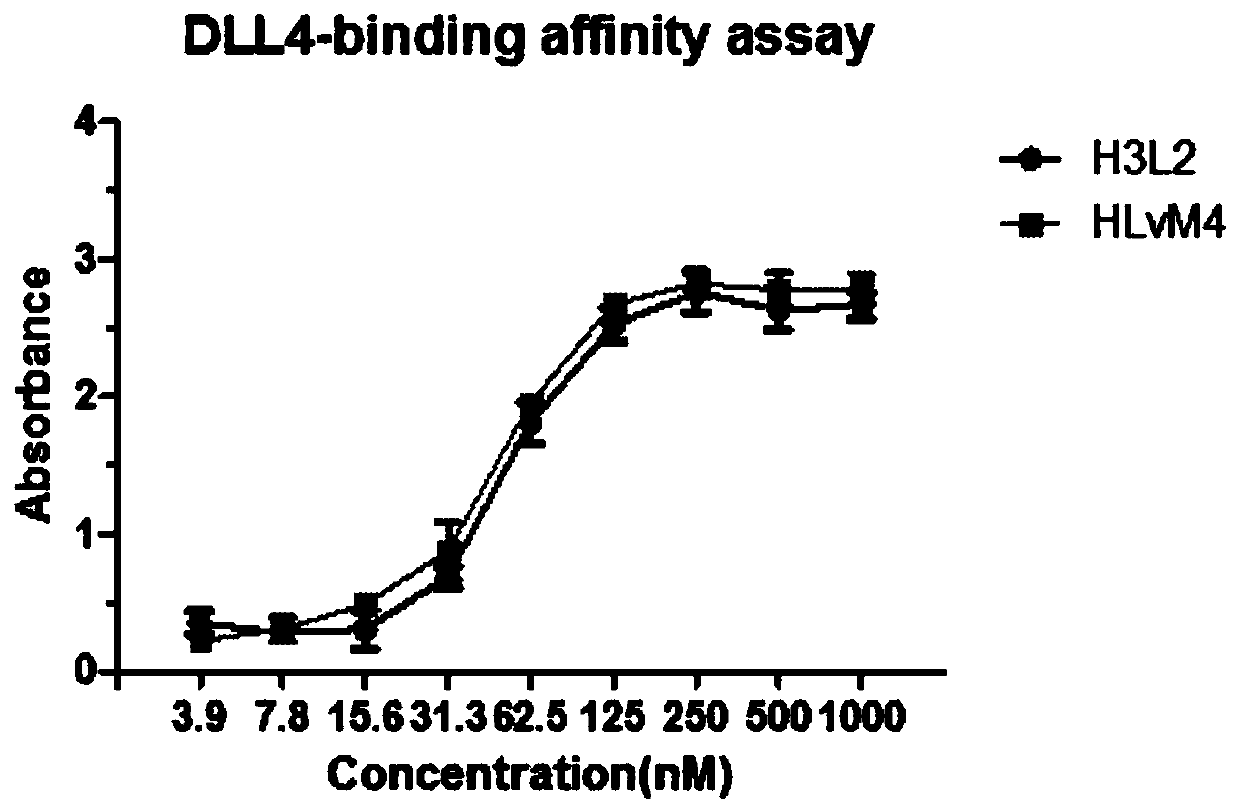
[0071] Affinity test of antibody drug conjugate HLvM4 and human DLL4 antigen: using ELISA method, add 1μg / mL DLL4 antigen to 96-well microtiter plate at 100μL per well, and coat overnight at 4℃; after washing the plate three times with PBS, it will be implemented The conjugates HLvM4 and H3L2 of Example 1, according to the blank group, the concentration gradients 0, 3.9, 7.8, 15.6, 31.3, 62.5, 125, 250, 500, 1000 nM were added to a 96-well plate, 37 ℃, incubated for 2 hours; After washing the plate three times with PBS, add goat anti-mouse IgG antibody coupled with horseradish peroxidase (HRP) at 37°C for 1 hour; after washing the plate three times with PBS, add TMB color developing solution at room temperature The reaction was kept in the dark for 20 minutes, and finally the stop solution was added to terminate the reaction. The microplate reader detected OD450-OD630.
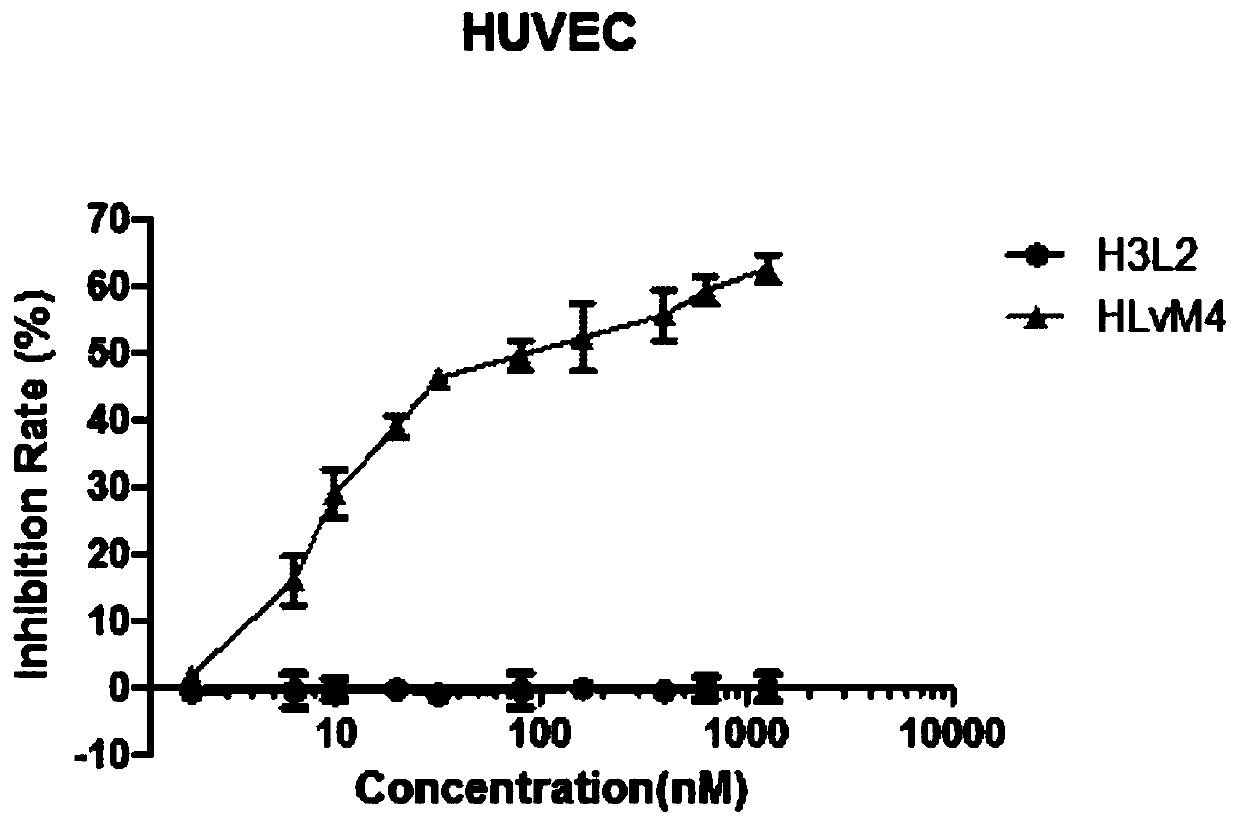
[0072] See the results of the experiment image 3 Compared with H3L2, HLvM4 has a slightly lower affinity for ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com