RNAi nano preparation and preparation method and application thereof in prevention and treatment of TMV
A nano preparation, chitosan nano technology, applied in genetic engineering technology and its application field, can solve the problems of lack, complicated preparation process of pharmaceuticals, poor dsRNA stability and delivery efficiency, etc., to reduce the expression amount and promote the practice and development. , The effect of not being easily degraded
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
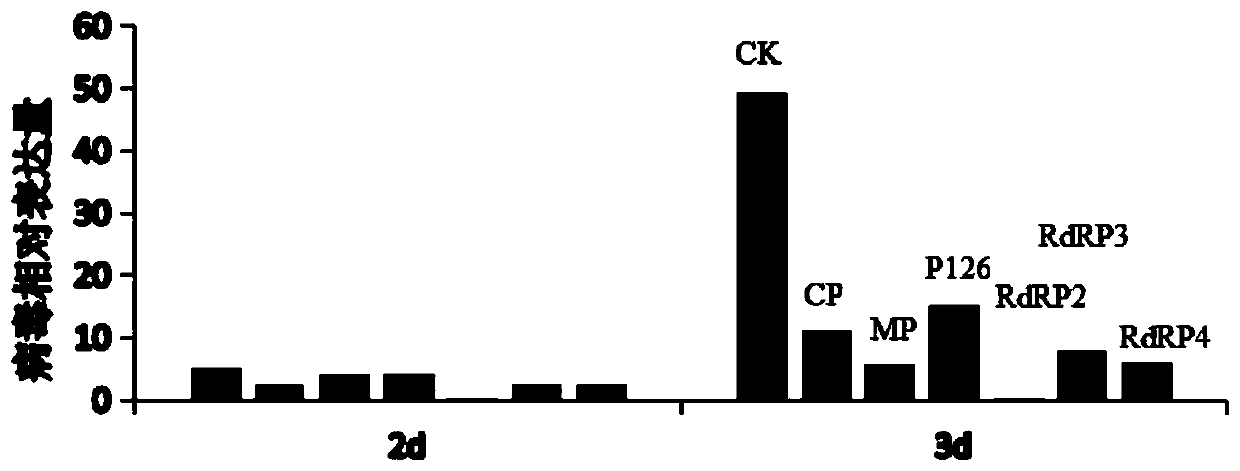
[0039] Example 1: This example provides a method for screening high-efficiency fragments and its application. After selecting six groups of candidate gene fragments and synthesizing dsRNA in vitro, their antiviral ability was evaluated by biological methods, and on this basis, dsRNA that efficiently degrades TMV-targeted nucleic acids was screened.
[0040] The six groups of candidate gene fragments of TMV are CP, MP, P126, RdRP2, RdRP3, and RdRP4, and their gene sequences are as shown in the sequence list: SEQ ID NO.4, 5, 6, 7, 1, and 8.
[0041] The dsRNA screening method for efficient prevention and treatment of TMV specifically comprises the following steps:
[0042] S1: extraction of total RNA from tobacco leaves infected with TMV. Reverse transcription was performed using the extracted total RNA as a template to obtain cDNA of TMV;
[0043] S2: Using cDNA as a template, design specific amplification primers for the six sequences of CP, MP, P126, RdRP2, RdRP3, and RdRP4, ...
Embodiment 2
[0118] Embodiment 2: the application of the high-efficiency dsRNA further screened in embodiment 1 in the prevention of TMV virus, the specific application method is as follows:
[0119] S1: Planting and transplanting of tobacco in the laboratory: the steps refer to S5-1 of Example 1, and the cultivated tobacco plant grows to an appropriate size.
[0120] S2: Injection of dsRNA and inoculation of TMV-30b: In this experiment, dsRNA was injected first, followed by inoculation of TMV-30b virus 24 hours later. Select benthic tobacco leaves of uniform size in advance and mark them. Dissolve 200 μg of dsRNA of MP and RdRP3 in 0.7ml H 2 O was injected into the leaves, and only 0.7ml H was injected into the leaves of the control group. 2 O. After 24 hours of inoculation, weigh a small amount of TMV-30b poison source leaves, grind them into juice in a mortar, add 40 times the volume of PBS buffer solution (pH 6.8) and mix well, and sprinkle a layer of 100-mesh quartz sand, use a co...
Embodiment 3
[0123] Embodiment 3: the preparation method of the nano-sized RNAi preparation for preventing and treating TMV, comprises the following steps:
[0124] (S1) The combination of chitosan nanomaterials and dsRNA, the specific operation steps are as follows:
[0125] S1-1: dissolving chitosan in glacial acetic acid to prepare chitosan solution A with a final concentration of 2 μg / μl;
[0126] S1-2: Slowly add TMV dsRNA solution with a concentration of 1 μg / μl into chitosan solution A, the volume ratio of chitosan and dsRNA is 10: (1-6). If the mass ratio is too low, many dsRNAs will not be combined with chitosan, which will eventually affect the effect of TMV virus prevention and treatment. If the ratio is too high, many chitosan nanomaterials will not be attached to dsRNA, resulting in unnecessary waste.
[0127] The dsRNA of RdRP3 gene sets 6 mixing ratios respectively, and chitosan glacial acetic acid solution: dsRNA volume ratio is respectively 10:1, 10:2, 10:3, 10:4, 10:5, 1...
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
| concentration | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com