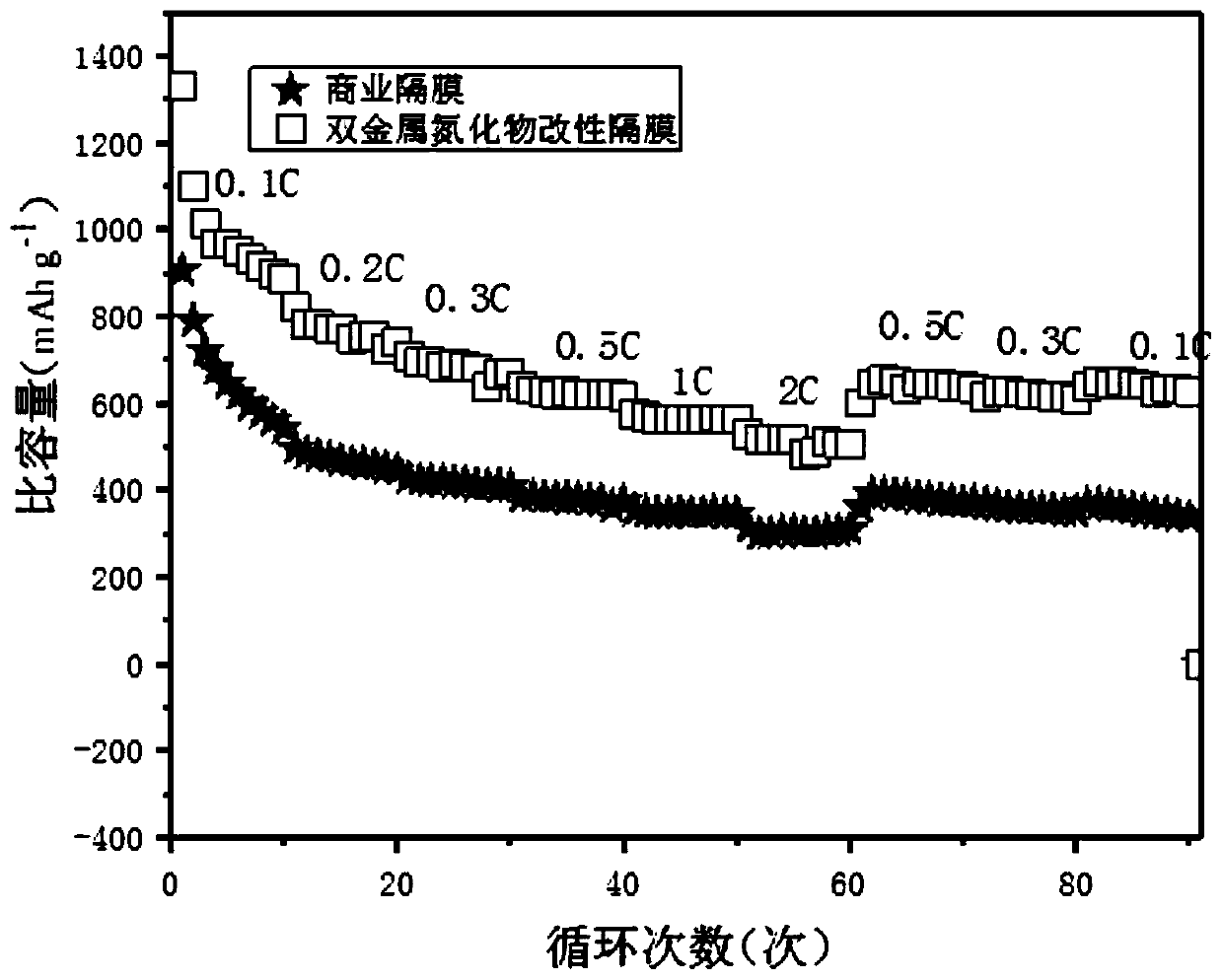
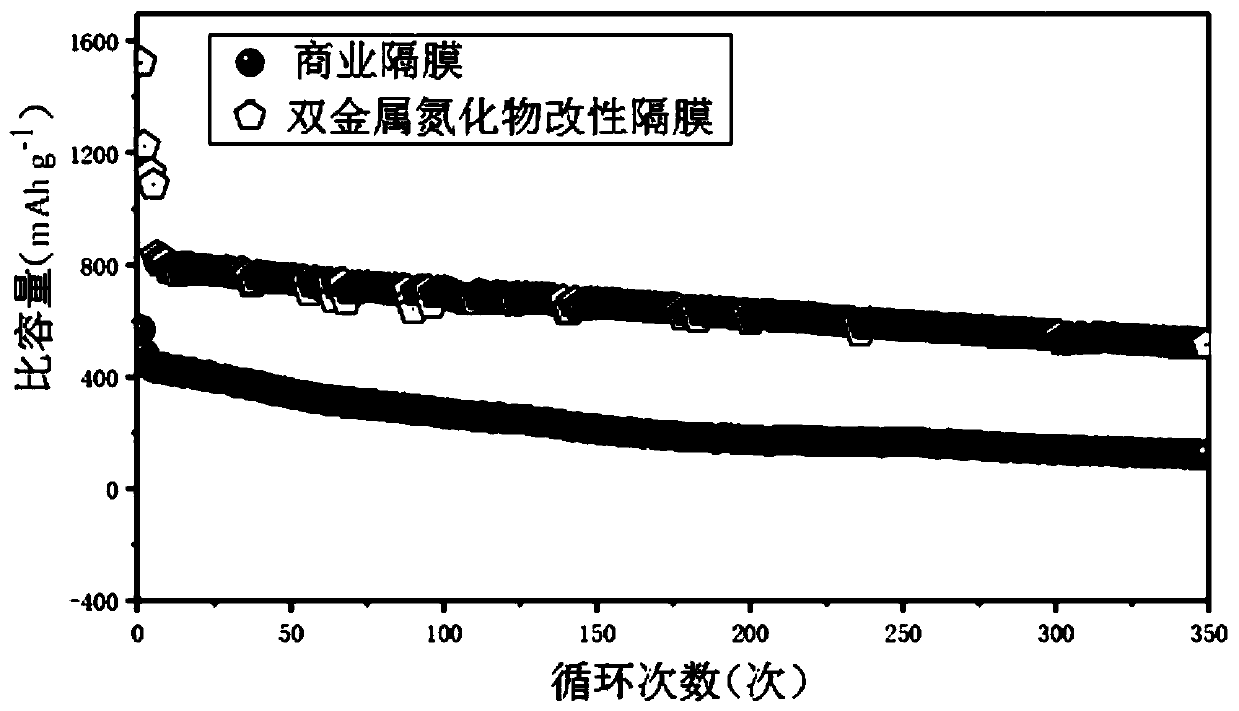
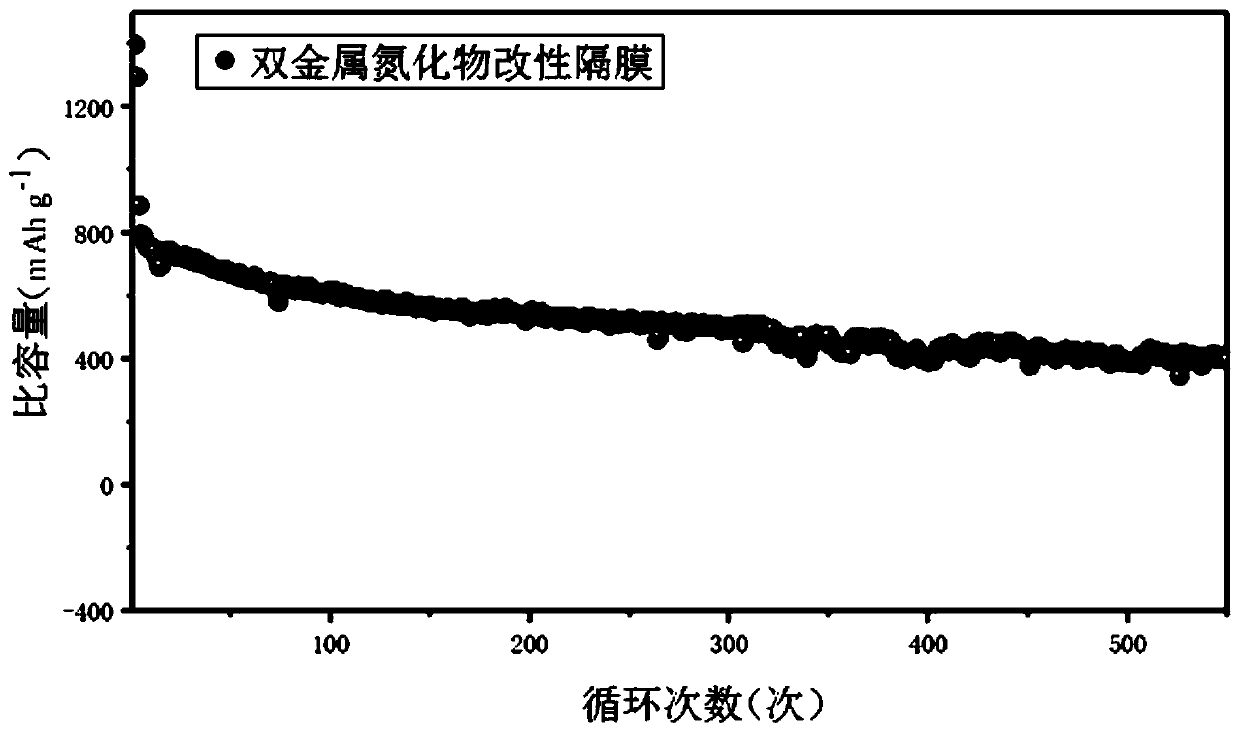
Bimetal nitride modified diaphragm as well as preparation method and application thereof
A nitride, bimetal technology, applied in the field of electrochemistry, can solve the problems of poor mechanical properties of products, affecting electrochemical properties, easy to pierce the separator, etc., to achieve improved mechanical properties, good lithium ion transport performance and low electrochemical impedance. , Improve the effect of high temperature resistance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0043] A double metal nitride modified diaphragm for lithium-sulfur batteries, the diaphragm includes a diaphragm base and a modified functional layer attached to the surface of the diaphragm base, the modified functional layer includes a double metal nitride and a binder, and the double metal nitrogen The compound is molybdenum nickel nitride, and the binder is a perfluorosulfonic acid polymer, which is prepared by the following steps:
[0044] Step 1: Dissolve 1g of ammonium molybdate and 0.1 of nickel sulfate in 10g of deionized water, then transfer to the reactor, and react at 100°C for 2h;
[0045] Step 2: centrifuge the reaction solution in step 1 at a speed of 6000r / min for 0.2h to obtain an intermediate product;
[0046] Step 3: Dry the intermediate product obtained in step 2 at 50°C for 10 hours, then transfer it to a tube furnace and heat it with ammonia gas at 400°C for 1 hour, wherein the molar ratio of the intermediate product to ammonia is 1:3;
[0047] Step 4: ...
Embodiment 2
[0053] A double metal nitride modified diaphragm for lithium-sulfur batteries, the diaphragm includes a diaphragm base and a modified functional layer attached to the surface of the diaphragm base, the modified functional layer includes a double metal nitride and a binder, and the double metal nitrogen The compound is molybdenum nickel nitride, and the binder is a perfluorosulfonic acid polymer, which is prepared by the following steps:
[0054] Step 1: Dissolve 1 g of ammonium tetrathiomolybdate and 0.6 nickel nitrate in 15 g of deionized water, transfer to a reaction kettle, and react at 160 ° C for 3 h;
[0055] Step 2: centrifuge the reaction solution in step 1 at a speed of 8000r / min for 0.3h to obtain an intermediate product;
[0056] Step 3: The intermediate product obtained in step 2 was dried at 60°C for 10 hours, then transferred to a tube furnace and heated at 500°C for 2 hours with ammonia gas, wherein the molar ratio of the intermediate product to ammonia was 1:2;...
Embodiment 3
[0059] A double metal nitride modified diaphragm for lithium-sulfur batteries, the diaphragm includes a diaphragm base and a modified functional layer attached to the surface of the diaphragm base, the modified functional layer includes a double metal nitride and a binder, and the double metal nitrogen The compound is molybdenum nickel nitride, and the binder is a perfluorosulfonic acid polymer, which is prepared by the following steps:
[0060] Step 1: Dissolve 1g of ammonium phosphomolybdate and 1g of nickel chloride in 30g of deionized water, transfer them to a reaction kettle, and react at 200°C for 6h;
[0061] Step 2: centrifuge the reaction solution in step 1 at a speed of 12000r / min for 0.3h to obtain an intermediate product;
[0062] Step 3: Dry the intermediate product obtained in step 2 at 60°C for 15 hours and transfer it to a tube furnace for heating at 600°C for 3 hours with ammonia gas, wherein the molar ratio of the intermediate product to ammonia is 1:2;
[0...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Aperture | aaaaa | aaaaa |
| Thickness | aaaaa | aaaaa |
| Thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com