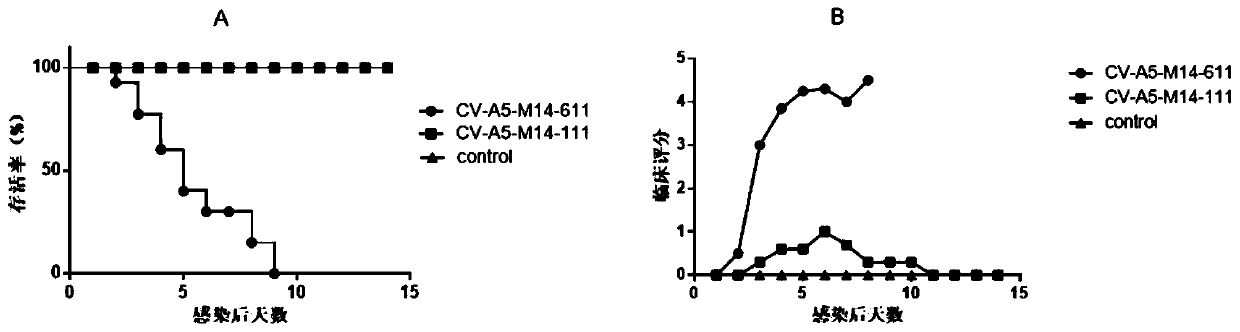
Coxsackie A group 5 virus strain capable of effectively infecting adult mice
An effective infection and virus strain technology, applied in the direction of positive-sense single-stranded RNA virus, virus, virus/phage, etc., to achieve the effect of strong growth ability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0091] Embodiment 1: Breeding of CV-A5-M14 mouse adapted strain
[0092] Cultivate the CV-A5-3487R4 / XY / CHN / 2017 virus strain in RD cells (the original strain is not a monoclonal strain, so it needs to be monoclonal after selection), and measure the virus titers on the RD cells according to the Reed-Muench method degree is 1x10 7 TCID 50 / ml.
[0093] Ten day-old SPF Kunming mice were infected with the virus strain by intracranial injection, 20 μL / mouse. The brains of mice on the verge of death were taken, weighed, and a 10% (W / V) homogenate was prepared, and the supernatant was harvested by centrifugation, and stored at -20°C. Then the harvested virus was continued to infect day-old suckling mice according to the same infection method, and was repeated three times.
[0094] Sequentially infect five-day-old suckling mice, repeat twice; seven-day-old mice, repeat twice.
[0095] Infect the 9-day-old suckling mice with the virus harvested from the 7-day-old infected sucklin...
Embodiment 2
[0097] Embodiment 2: CV-A5-M14 virus strain purification
[0098] 1. Plaque purification of CV-A5-M14 virus clone
[0099] (1) The CV-A5-M14 virus solution was serially diluted 10 times, and the dilution range was 10 -1 ~10 -6 .
[0100] (2) Take a 6-well plate with RD cell confluence of 90%-95%, add 200 μL / well of diluted virus suspension, and incubate for 2 hours.
[0101] (3) After incubation, discard the virus solution. After the pre-configured 2X low-melting point agarose is melted, mix it with 2X MEM virus maintenance solution 1:1 and spread it in a 6-well plate, 2ml / well.
[0102] (4) Microscopically check the CPE condition of each well, pick a CPE-positive clone from the well corresponding to the maximum dilution, suspend the virus in 300 μL virus maintenance solution, and blow and mix well.
[0103] (5) Take 100 μL of the harvest solution for ten-fold dilution, and the dilution range is 10 -1 ~10 -3 , repeat the above steps for three consecutive plaque purifica...
Embodiment 3
[0109] Example 3: CV-A5-M14-611 and CV-A5-M14-111 Whole Genome Sequence Determination
[0110] 1. Viral RNA extraction
[0111] Use the Sangon Bioengineering (Shanghai) Co., Ltd. Column Viral RNA Extraction and Purification Kit (Product No.: B518667) to extract and purify viral RNA.
[0112] 2. cDNA synthesis by reverse transcription
[0113] The reverse transcription synthesis cDNA reaction system is as follows
[0114]
[0115] (1) Add the reagents into the PCR tube, mix well, place in the PCR instrument, react at 65°C for 5min, and then quickly place it on ice to quench.
[0116] (2) Add reagents to the above reaction solution, mix well and place in PCR instrument, 30°C, 15min; 42°C, 60min; 70°C, 15min.
[0117] 3. PCR amplification
[0118] (1) Amplification primers: CV-A5 full sequence determination and extension primers are shown in Table 1, three pairs of primers are used for PCR (1F5R, 6F8R, 9F10R), and others are primers for sequencing.
[0119] Table 1: CV-A5...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Titer | aaaaa | aaaaa |
| Titer | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com