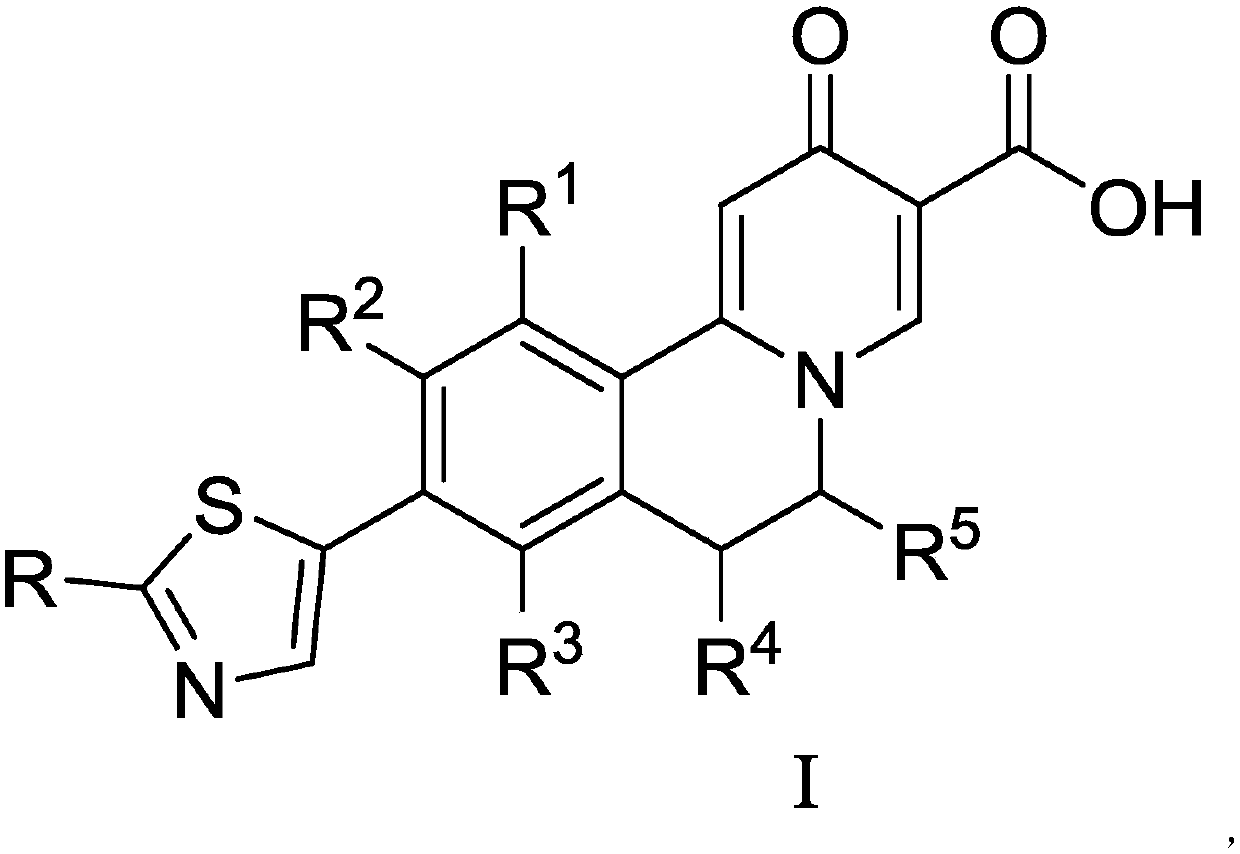
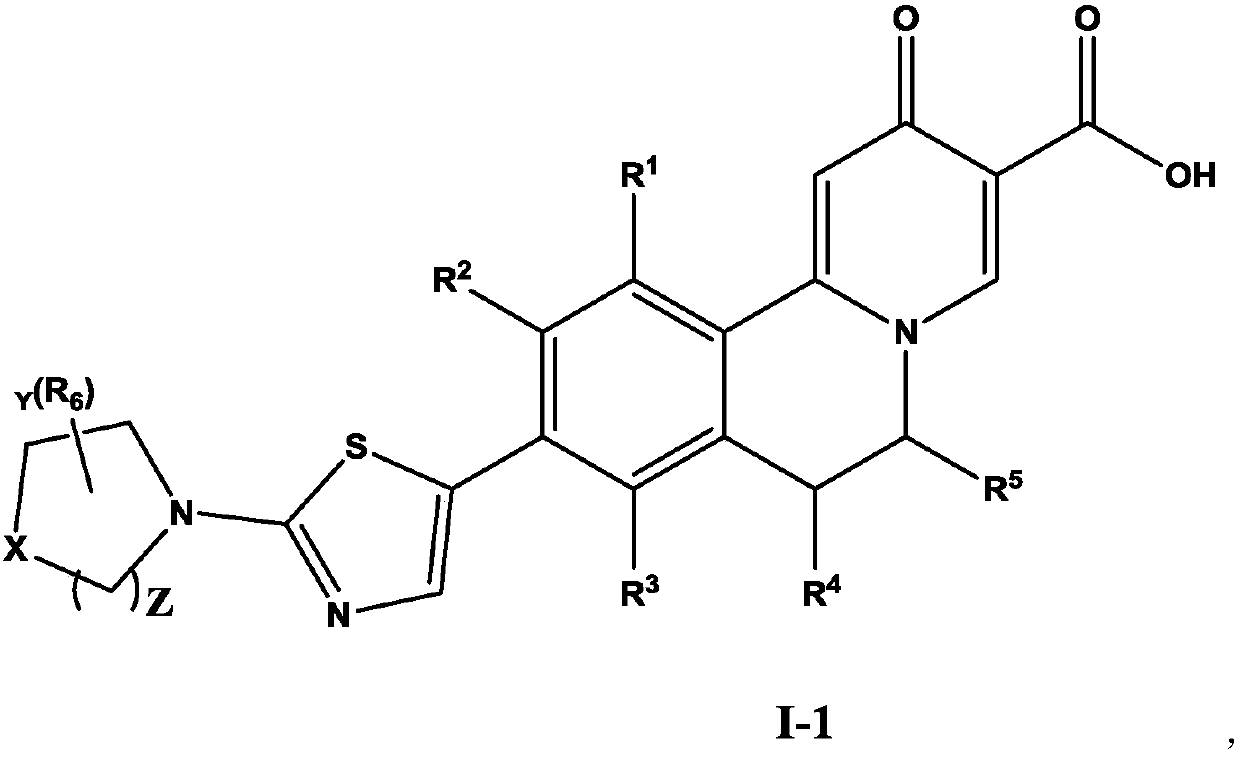
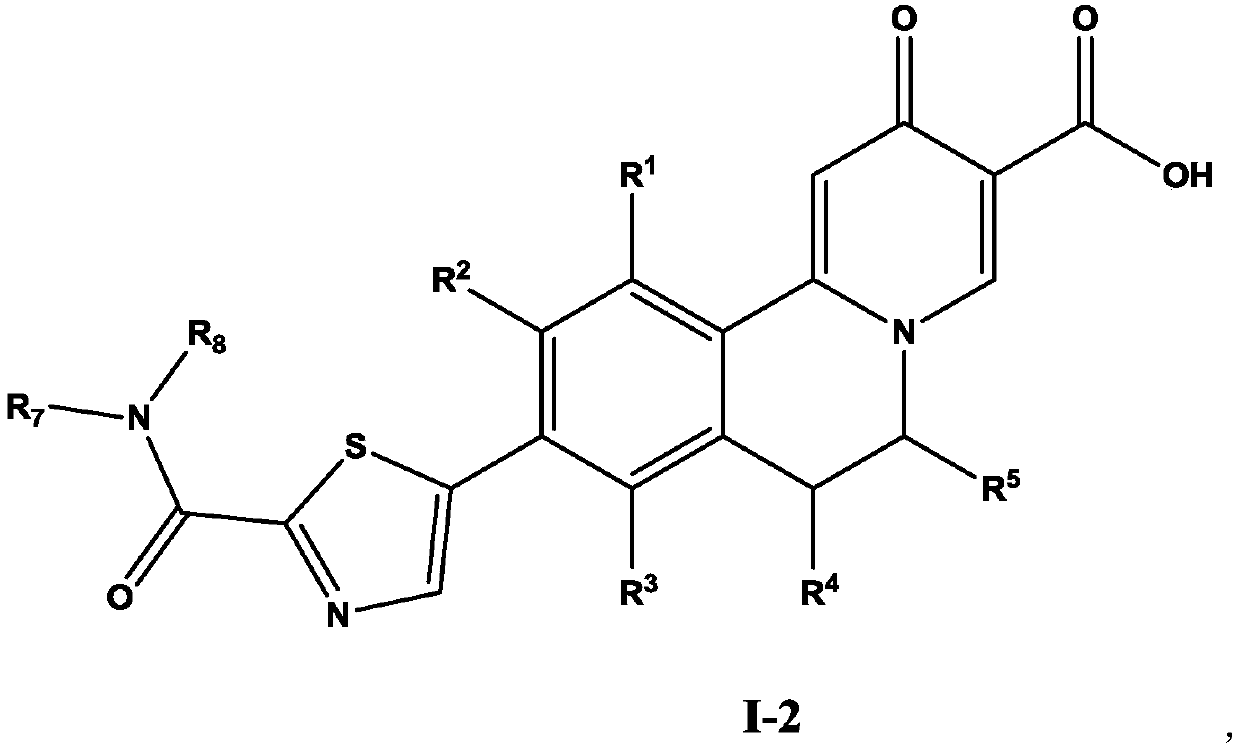
Dihydroisoquinoline compound
A technology of compounds and hydrates, applied in the fields of organic chemistry, organic active ingredients, antiviral agents, etc., can solve the problem that the activity of compounds is not very high
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0339] 6-tert-butyl-9-[2-(4-hydroxypiperidin-1-yl)thiazol-5-yl]-10-methoxy-2-oxo-6,7-dihydro-2H-pyridine And[2,1-a]isoquinoline-3-carboxylic acid
[0340]
[0341] Step 1a: Preparation of 6-tert-butyl-10-methoxy-2-oxo-9-(4,4,5,5-tetramethyl-1,3,2-dioxaborolane- 2-yl)-6,7-dihydro-2H-pyrido[2,1-a]isoquinoline-3-carboxylic acid ethyl ester
[0342]
[0343]Under nitrogen protection, to 9-bromo-6-tert-butyl-10-methoxy-2-oxo-6,7-dihydro-2H-pyridine[2,1-a]isoquinoline-3-carboxylic acid Add double pinacol borate (3.5g, 13.81mmol) to ethyl ester (see Example 8 in WO 2018130152 for the preparation method) (3.0g, 6.91mmol) in 1,4-dioxane (50mL) solution , potassium acetate (1.4 g, 13.81 mmol) and [1,1'-bis(diphenylphosphino)ferrocene]palladium dichloride (505 mg, 0.69 mmol). The mixture was stirred at 100° C. for 1 hour. The mixture was diluted with dichloromethane (100 mL), then washed with water (100 mL) and saturated brine (100 mL), then dried over anhydrous sodium sulfate, ...
Embodiment 2
[0354] 6-tert-butyl-9-{2-[4-(hydroxymethyl)piperidin-1-yl]thiazol-5-yl}-10-methoxy-2-oxo-6,7-dihydro -2H-pyrido[2,1-a]isoquinoline-3-carboxylic acid
[0355]
[0356] Step 2a: Preparation of [1-(5-bromothiazol-2-yl)piperidin-4-yl]methanol
[0357]
[0358] 2,5-dibromothiazole (100mg, 0.41mmol) and piperidin-4-ylmethanol (57mg, 0.49mmol) were prepared according to a method similar to Example 1 step 1b to obtain a white solid [1-(5-bromothiazole -2-yl)piperidin-4-yl]methanol (51 mg).
[0359] Step 2b: Preparation of 6-tert-butyl-9-{2-[4-(hydroxymethyl)piperidin-1-yl]thiazol-5-yl}-10-methoxy-2-oxo-6, 7-Dihydro-2H-pyrido[2,1-a]isoquinoline-3-carboxylic acid ethyl ester
[0360]
[0361] 6-tert-butyl-10-methoxy-2-oxo-9-(4,4,5,5-tetramethyl-1,3,2-dioxaborolan-2-yl )-6,7-dihydro-2H-pyrido[2,1-a]isoquinoline-3-carboxylic acid ethyl ester (100mg, 0.21mmol) and [1-(5-bromothiazol-2-yl)piper Pyridin-4-yl]methanol (58mg, 0.21mmol) was prepared according to a method similar t...
Embodiment 3
[0366] 9-[2-(azetidin-1-yl)thiazol-5-yl]-6-tert-butyl-10-methoxy-2-oxo-6,7-dihydro-2H-pyridine And[2,1-a]isoquinoline-3-carboxylic acid
[0367]
[0368] Step 3a: Preparation of 2-(azetidin-1-yl)-5-bromothiazole
[0369]
[0370] 2,5-dibromothiazole (300mg, 1.23mmol) and azetidine (85mg, 1.48mmol) were prepared according to a method similar to Example 1 step 1b to obtain white solid 2-(azetidine-1 -yl)-5-bromothiazole (55mg).
[0371] Step 3b: Preparation of 9-[2-(azetidin-1-yl)thiazol-5-yl]-6-tert-butyl-10-methoxy-2-oxo-6,7-dihydro -2H-Pyrido[2,1-a]isoquinoline-3-carboxylic acid ethyl ester
[0372]
[0373] 6-tert-butyl-10-methoxy-2-oxo-9-(4,4,5,5-tetramethyl-1,3,2-dioxaborolan-2-yl )-6,7-dihydro-2H-pyrido[2,1-a]isoquinoline-3-carboxylic acid ethyl ester (100mg, 0.21mmol) and 2-(azetidin-1-yl)- 5-Bromothiazole (50mg, 0.23mmol) was prepared according to a method similar to Example 1 step 1c to obtain a yellow solid 9-[2-(azetidin-1-yl)thiazol-5-yl]-6-tert Butyl...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com