Preparation method of vilanterol
The technology of a compound and a reducing agent, which is applied in the field of preparation of vilanterol, can solve the problems of being unsuitable for industrial scale-up production, difficult to separate and purify, and difficult to synthesize large-scale β-hydroxyamine compounds.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
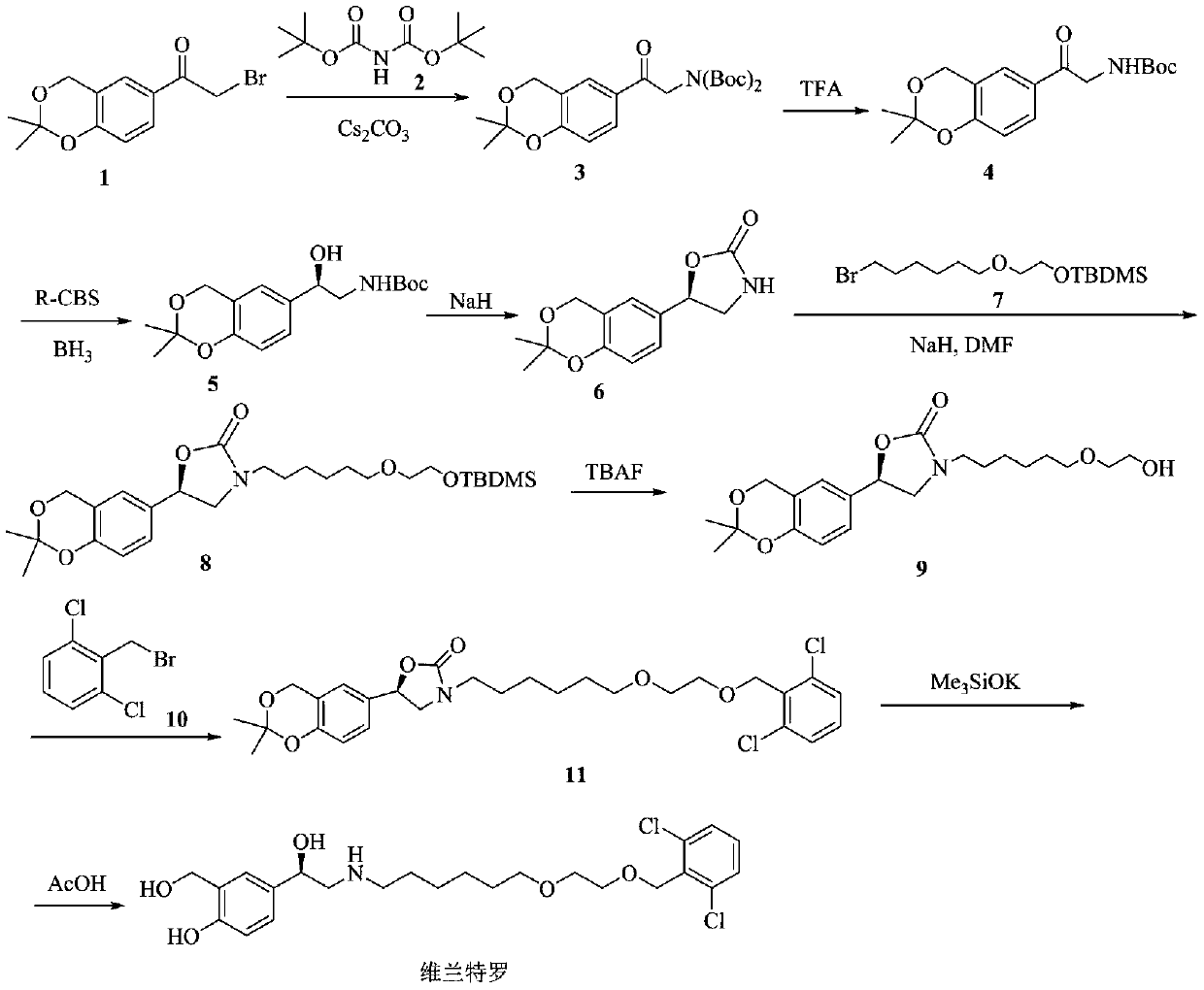
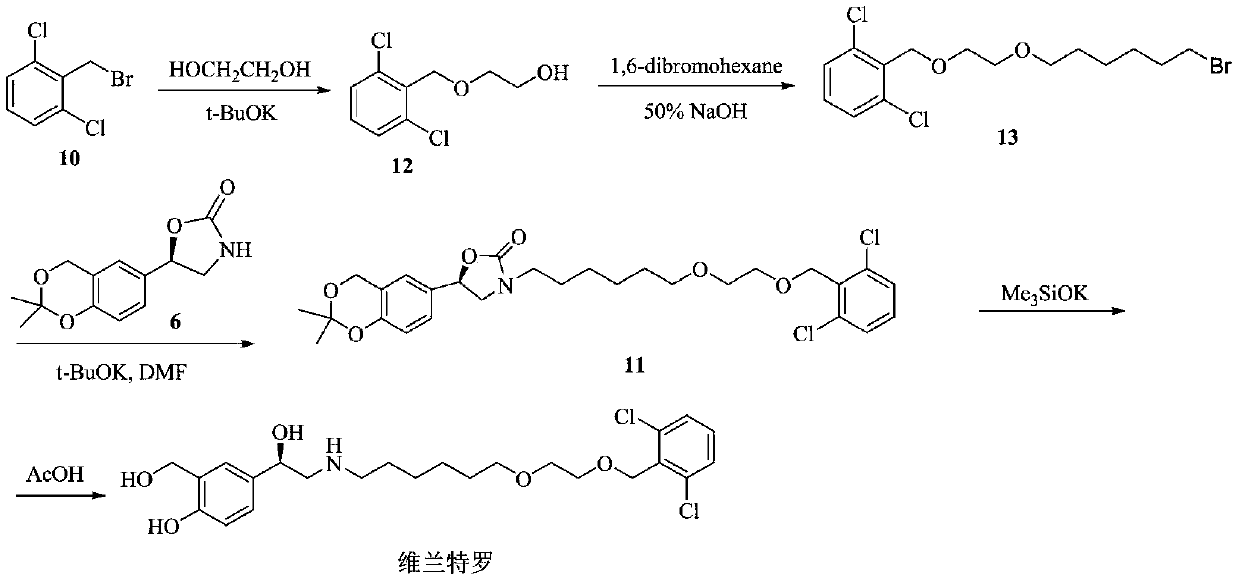
Image
Examples
Embodiment 1
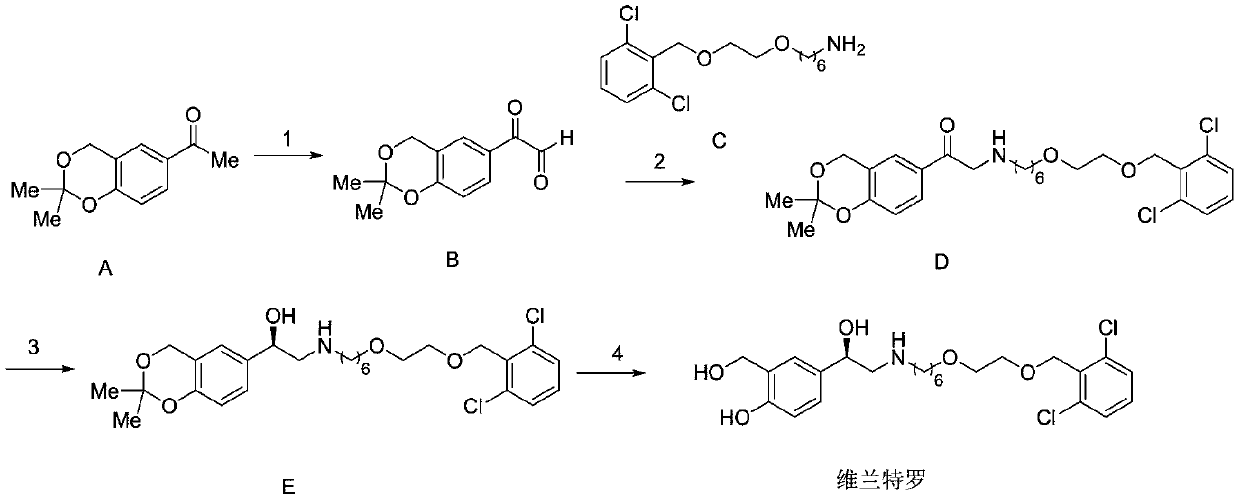
[0057] Embodiment 1: the preparation of compound B
Embodiment 1-1
[0059] Add 10g of compound A, 80mL of 1,4-dioxane, 10.8g of selenium dioxide and 2mL of water into a 250mL three-necked flask, stir, and control the temperature at 95-100°C for reaction. After the reaction was complete, the reaction system was cooled to room temperature, allowed to stand, filtered, and the filtrate was collected. The solvent was removed in vacuo to obtain 9.4 g of compound B, the molar yield was 88.0%, and the HPLC purity was 93.7%.
Embodiment 1-2
[0061] Add 10g of compound A, 100mL of ethanol, 8.5g of selenium dioxide and 3mL of water into a 250mL three-necked flask, stir, and control the temperature at 75-80°C for reaction. After the reaction was complete, the reaction system was cooled to room temperature, allowed to stand, filtered, and the filtrate was collected. The solvent was removed in vacuo to obtain 9.6 g of compound B, the molar yield was 89.9%, and the HPLC purity was 92.9%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com