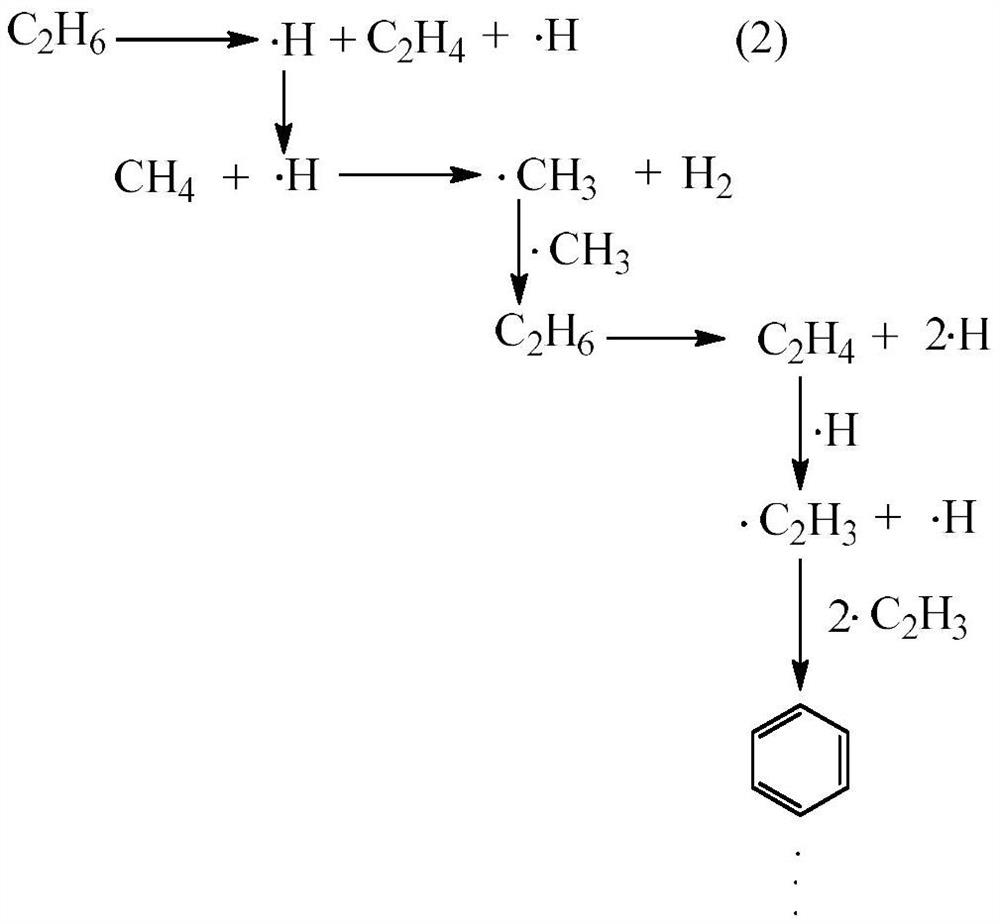
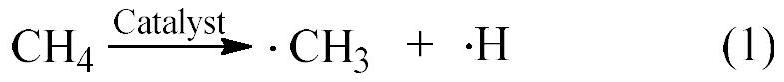A method for the co-catalytic conversion of methane and ethane into olefins, aromatics and hydrogen
A technology for producing ethane and methane, which is applied in chemical instruments and methods, catalyst activation/preparation, hydrogen/synthesis gas production, etc., and can solve the problems of harsh catalyst preparation conditions, low selectivity, and large catalyst bed pressure drop.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0062] 1. Preparation of catalytic reactor (thin layer thickness and active component content need to be indicated)
[0063] The preparation method of the lattice doped catalyst includes chemical vapor deposition (MCVD) coating solid-phase doping technology or solid-liquid phase sol-gel combined with high-temperature melting coating technology. Membrane catalysts are marked as:
Embodiment 1
[0066] Modified Chemical Vapor Deposition (MCVD)
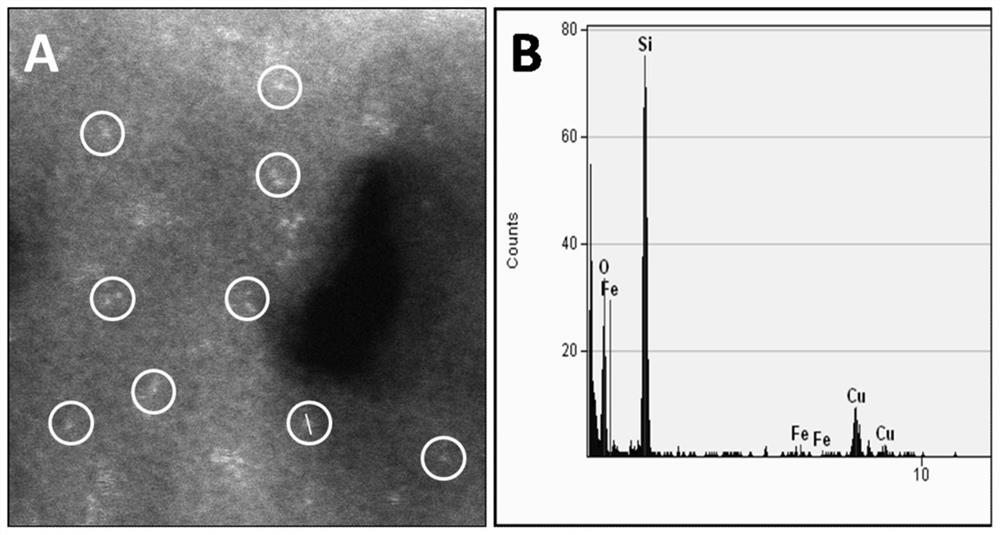
[0067] Use 30mL / min of high-purity oxygen to SiCl 4 Liquid and FeCl under saturated vapor pressure at 350°C using 200mL / min high-purity helium 3 The gas is brought into the high-temperature MCVD device, and the contact surface of the quartz tube (wall thickness 1.5mm) with an outer diameter of 20mm and a length of 100mm is SiCl at 1600°C 4 and FeCl 3 After 10 min of oxide deposition, Fe-doped SiO was obtained 2 The powder material is then melted for 40 minutes at a temperature of 1980°C under a 2bar high-purity helium atmosphere to form a thin layer of dopant with a thickness of 100nm on the contact surface of the reactor, and then naturally cooled to obtain a diameter of 20mm and a length of 100mm of Catalytic quartz reactor, wherein Fe doping amount is 0.05wt.%.
Embodiment 2
[0069] Modified Chemical Vapor Deposition (MCVD)
[0070] Use 30mL / min of high-purity oxygen to SiCl 4 Liquid and FeCl under saturated vapor pressure at 350°C using 650mL / min high-purity helium 3 The gas is brought into the high-temperature MCVD device, and the inner wall of the quartz tube (wall thickness 1.5mm) with an outer diameter of 20 mm and a length of 100 mm is heated at 1600 ° C by SiCl 4 and FeCl 3 After 10 min of oxide deposition, Fe-doped SiO was obtained 2 The powder material is then melted for 40 minutes at a temperature of 1980°C under a 2bar high-purity helium atmosphere to form a thin layer of dopant with a thickness of 100nm on the contact surface of the reactor, and then naturally cooled to obtain a diameter of 20mm and a length of 100mm of Catalytic quartz reactor, wherein Fe doping amount is 0.1wt.%.
PUM
| Property | Measurement | Unit |
|---|---|---|
| thickness | aaaaa | aaaaa |
| thickness | aaaaa | aaaaa |
| thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com