Polymer drug-loaded film for artificial cochlear implant, artificial cochlear drug-loaded electrode and preparation method
A technology of cochlear implant and polymer carrier, applied in the field of medical devices, can solve problems such as damage to auditory reconstruction, residual hearing, etc., and achieve the effects of facilitating hearing protection, inhibiting the formation of local fibrous tissue, and simple process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
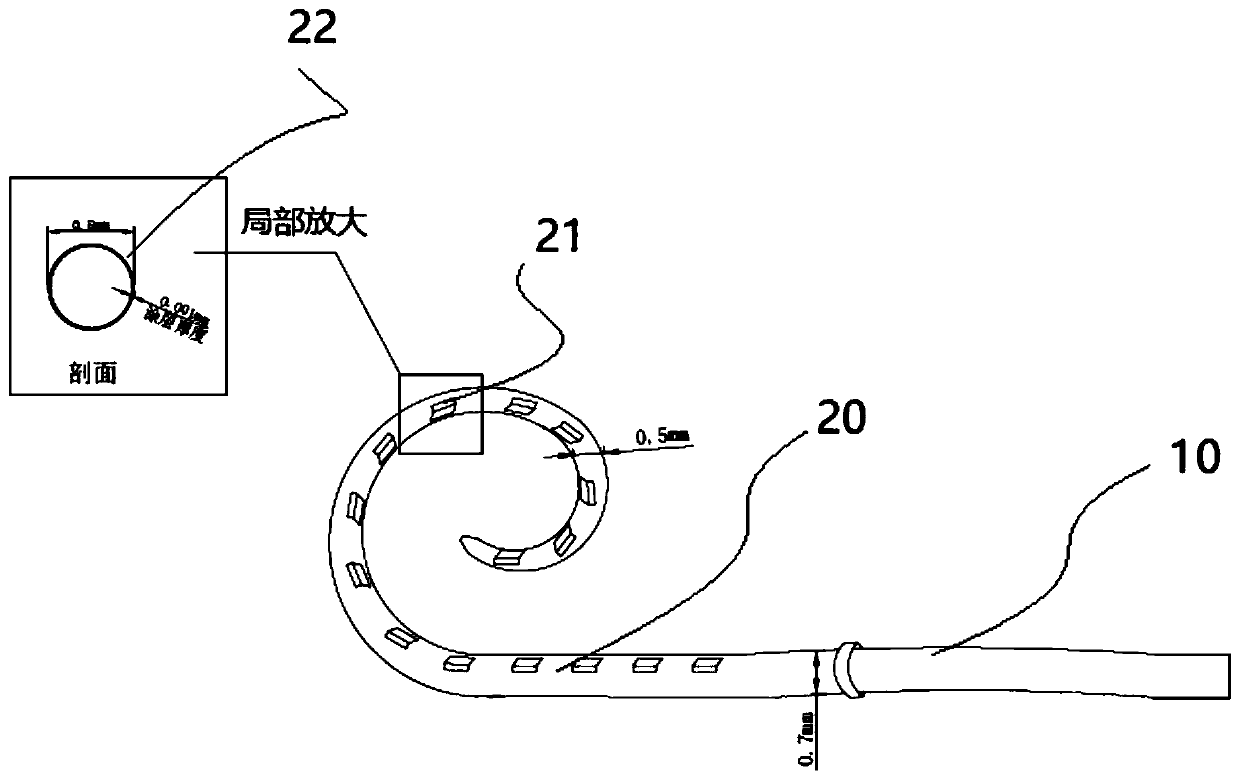
[0038] Taking the most commonly used dexamethasone sodium phosphate (DSP) as an example, the doctor decided that the electrode drug loading plan for this operation is to add 10% DSP to the PLGA chloroform solution with a mass fraction of 5%. That is, under this condition, the surface of each electrode implant segment will be covered with about 800 μg of PLGA film, which contains 80 μg of DSP, and they will be implanted into the inner ear together.
[0039] At room temperature, prepare the corresponding amount of drug and PLGA chloroform solution in a brown light-proof wide-mouth glass container with a cover (such as a Petri dish) according to the requirements of this drug loading protocol, and stir evenly to obtain a precursor solution for later use.
[0040] By holding the cochlear implant clamping device, the operator assists to completely immerse the electrode implanted segment of the cochlea in the above-mentioned prepared precursor solution. After 1-3 seconds, the fully co...
Embodiment 2
[0044] In the PLGA chloroform solution with a mass fraction of 6%, add DSP accounting for 15% of the PLGA mass ratio, that is, under this condition, the surface of each electrode implanted segment will cover about 1000 μg of PLGA film, which contains the mass of DSP 150μg, implanted together in the inner ear.
[0045] At room temperature, prepare the corresponding amount of drug and PLGA chloroform solution in a brown light-proof wide-mouth glass container with a cover (such as a Petri dish) according to the requirements of this drug loading protocol, and stir evenly to obtain a precursor solution for later use.
[0046] By holding the cochlear implant clamping device, the operator assists to completely immerse the electrode implanted segment of the cochlea in the above-mentioned prepared precursor solution. After 1-3 seconds, the fully covered precursor solution is slowly taken out perpendicular to the liquid surface After the precursor solution is naturally leveled and dried...
Embodiment 3
[0050] The method is the same as in Example 1, except that the formulation of the precursor solution is different.
[0051] The formulation of the precursor solution in this embodiment is: in the chloroform solution of PLGA with a mass fraction of 4%, cytarabine hydrochloride with a mass ratio of 1% of PLGA is added.
[0052] The prepared polymer drug-loaded coating film is smooth, uniform and thin, and its surface contact angle is about 76.60°, which is significantly smaller than that before coating.
PUM
| Property | Measurement | Unit |
|---|---|---|
| thickness | aaaaa | aaaaa |
| viewing angle | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com