Quality control method of artemisia argyi and indigofera tinctoria granules
A quality control method and artemisia board technology, applied in measuring devices, instruments, scientific instruments, etc., can solve problems such as unfavorable quality control, complicated extraction processing procedures, poor repeatability, etc., and achieve scientific and reasonable quality control indicators and comprehensive testing items. Complete, easy-to-use sample handling
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0042] A quality control method for Haobanqing granules, comprising the following steps:
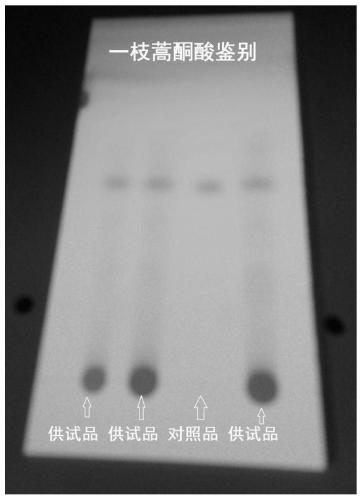
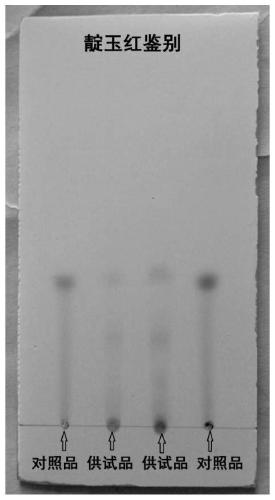
[0043] (1) Qualitatively identify the characteristic active ingredient of Artemisia annua L. in the drug, Artemisinic acid, by thin-layer chromatography: take 5 g of finely ground powder of Artemisia Banqing granules, add 40 mL of ethanol, ultrasonically treat for 20 min, filter, and take the filtrate. Evaporate to dryness, add 3 mL of ethanol again to dissolve the evaporated product, and use it as the test solution. Take another artemisinic acid reference substance, add ethanol to make a solution containing 0.08mg per 1mL, as the reference substance solution.
[0044] According to the thin-layer chromatography (Appendix 0502) test, draw 5 μl of each of the above two solutions, and place them on the same silica gel GF254 thin-layer plate. 15:8:1) is a developing agent, unfold, take out, dry, and inspect under ultraviolet light. In the chromatogram of the test substance, a fluorescent s...
Embodiment 2
[0057] A quality control method for Haobanqing granules, comprising the following steps:
[0058] (1) Qualitative identification of the characteristic active ingredient of Artemisia annua L. in the drug, Artemisinic acid, by thin-layer chromatography: take 5 g of finely ground powder of Artemisia Banqing granules, add 20 mL of methanol, ultrasonically treat for 60 min, filter, and take the filtrate. Evaporate to dryness, add 1 mL of methanol again to dissolve the evaporated product, and use it as the test solution. Another reference substance of artemisinic acid was taken, and methanol was added to make a solution containing 0.05 mg per 1 mL, which was used as the reference substance solution.
[0059] According to the thin-layer chromatography (Appendix 0502) test, draw 5 μl of each of the above two solutions, and place them on the same silica gel GF254 thin-layer plate respectively. 30:5:1) as a developing agent, unfold, take out, dry, and inspect under an ultraviolet light...
Embodiment 3
[0070] (1) Qualitatively identify the characteristic active ingredient of Artemisia annua L. - Artemisinic acid by thin-layer chromatography: take 5 g of finely ground powder of Artemisia Banqing granules, add 80 mL of ethanol, ultrasonically treat for 10 min, filter, and take the filtrate Evaporate to dryness, add 5 mL of ethanol again to dissolve the evaporated product, and use it as the test solution. Take another artemisinic acid reference substance, add ethanol to make a solution containing 0.15mg per 1mL, as the reference substance solution.
[0071] According to the thin-layer chromatography (Appendix 0502) test, draw 5 μl of each of the above two solutions, and place them on the same silica gel GF254 thin-layer plate. 10:15:1) is the developing agent, unfold it, take it out, dry it, and inspect it under a UV light. In the chromatogram of the test substance, a fluorescent spot is displayed at the position corresponding to the chromatogram of the reference substance.
...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com