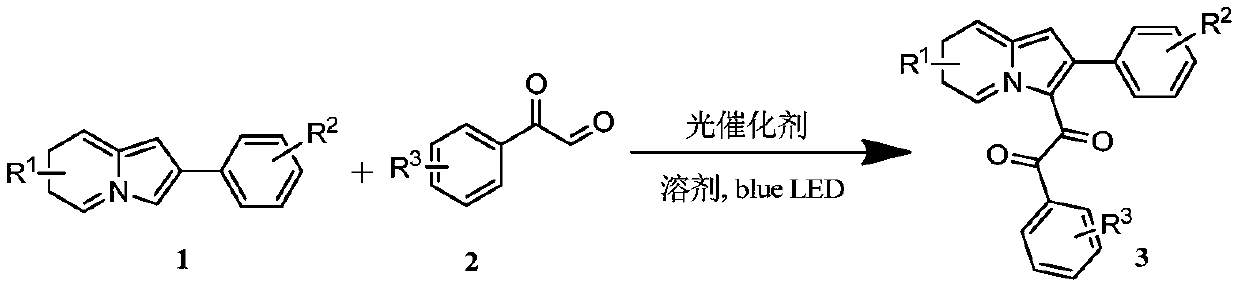
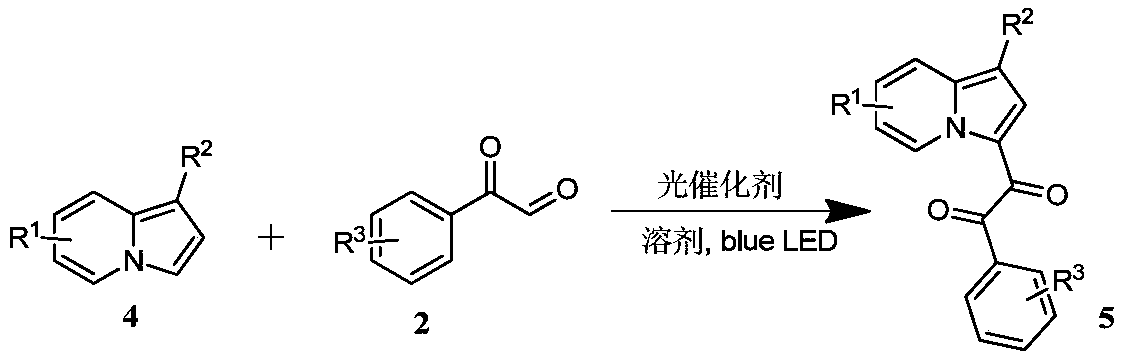
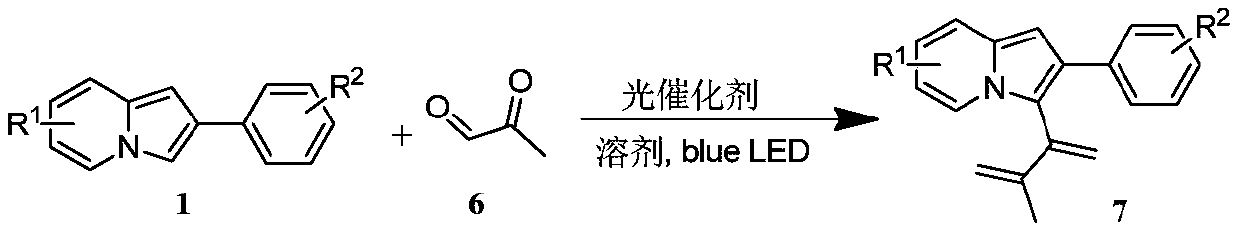
Preparation method of indolizine cyclo-1, 2-diketone with fluorescence activity and derivative thereof
A technology of indolezine and phenylindolezine, which is applied in the field of preparation of indolezine ring-1,2-dione and its derivatives, and can solve the problems of harsh conditions, narrow scope of application of substrates, low yield, etc. problems, to achieve the effect of easy operation, wide application range and simple operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0042] Sample preparation and testing:
[0043] Put 19.3mg (0.1mmol) of 2-phenylindolezine and 1.1mg (0.001mmol) of Rose Bengal into a 25ml test tube with a stirring bar, add 1mL of dimethyl sulfoxide at room temperature, and then add 20.1 mg (0.15 mmol) of 2-oxo-2-phenylacetaldehyde, stirred for 12 hours under blue light irradiation, spin-dried and flash column chromatography to obtain the target product.
[0044]
[0045] Result analysis:
[0046] (1) Proton NMR spectrum: 1 H NMR (400MHz, Chloroform-d) δppm10.09(d, J=6.9Hz, 1H), 7.67-7.62(m, 2H), 7.59(d, J=8.7Hz, 1H), 7.50(t, J= 7.3Hz, 1H), 7.33(q, J=8.7, 8.0Hz, 3H), 7.14(d, J=7.3Hz, 1H), 7.07(t, J=8.7Hz, 3H), 7.00(t, J= 7.5Hz,2H),6.53(s,1H).
[0047] (2) Carbon NMR spectrum: 13 C NMR (100MHz, Chloroform-d) δppm 192.4, 183.2, 142.2, 139.4, 134.2, 134.0, 133.6, 130.4, 129.6, 129.6, 128.4, 128.0, 127.6, 126.6, 118.8, 118.5, 114.9, 106.
[0048] (3) High resolution mass spectrometry: HRMS MALDI (m / z): calcd for C 22 h...
Embodiment 2
[0051] Sample preparation and testing:
[0052] Put 14.2mg (0.1mmol) of 1-cyanindolezine and 1.1mg (0.001mmol) of Rose Bengal into a 25ml test tube with a stirring bar, add 1mL of dimethyl sulfoxide at room temperature, and then add 20.1 mg (0.15 mmol) of 2-oxo-2-phenylacetaldehyde, stirred for 12 hours under blue light irradiation, spin-dried and flash column chromatography to obtain the target product.
[0053]
[0054] Result analysis:
[0055] (1) Proton NMR spectrum: 1 H NMR (400MHz, Chloroform-d) δppm 9.95(d, J=7.0Hz, 1H), 8.03(d, J=6.9Hz, 2H), 7.84(d, J=8.8Hz, 1H), 7.75(s, 1H), 7.65(t, J=7.4Hz, 1H), 7.60-7.56(m, 1H), 7.50(t, J=7.7Hz, 2H), 7.26-7.21(m, 1H).
[0056] (2) Carbon NMR spectrum: 13 C NMR (100MHz, Chloroform-d) δppm 191.8, 181.6, 141.8, 134.9, 132.9, 130.3, 130.3, 129.9, 129.3, 129.0, 120.6, 117.8, 117.0, 114.4, 86.9.
[0057] (3) High resolution mass spectrometry: HRMS MALDI (m / z): calcd for C 17 h 10 N 2 o 2 [M+Na] + :297.0635,found:297.0644.
...
Embodiment 3
[0060] Sample preparation and testing:
[0061] Put 19.3mg (0.1mmol) of 2-phenylindolezine and 1.1mg (0.001mmol) of Rose Bengal into a 25ml test tube with a stirring bar, add 1mL of dimethyl sulfoxide at room temperature, and then add 10.8 mg (0.15 mmol) of methylglyoxal, stirred for 12 hours under blue light irradiation, spin-dried and flash column chromatography to obtain the target product.
[0062]
[0063] Result analysis:
[0064] (1) Proton NMR spectrum: 1 H NMR (400MHz, Chloroform-d) δppm 9.84 (d, J = 6.9Hz, 1H), 7.56 (d, J = 8.4Hz, 1H), 7.39 (dd, J = 4.3, 2.2Hz, 3H), 7.36- 7.34(m, 2H), 7.29(d, J=7.4Hz, 1H), 6.97(t, J=6.8Hz, 1H), 6.57(d, J=1.5Hz, 1H), 2.15(s, 3H).
[0065] (2) Carbon NMR spectrum: 13 C NMR (100MHz, Chloroform-d) δppm 200.4, 183.4, 141.6, 139.3, 135.3, 129.9, 129.1, 128.4, 128.3, 126.3, 118.5, 117.2, 114.70, 105.7, 26.3.
[0066] (3) High resolution mass spectrometry: HRMS MALDI (m / z): calcd for C 17 h 13 NO 2 [M+Na] + :286.0839,found:286.08...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com