Method for catalytically synthesizing diethyl maleate by using acidic ionic liquid
A technology of diethyl maleate and acidic ionic liquid, which is applied in the field of organic synthesis fine chemicals, can solve problems such as increasing production cost, large amount of water, side reactions, etc., and achieves improved reaction conversion rate, high catalytic activity, and thermal stability. high sex effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
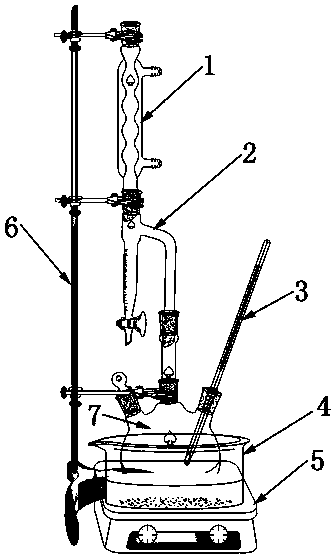
[0028] as per figure 1 The structure shown builds the reaction apparatus.
[0029] Add 9.80 g (0.10 mol) of maleic anhydride, 18.43 g (0.40 mol) of absolute ethanol, 15 mL of cyclohexane (as a water carrier) and 3.22 g (0.02 mol) of acidic ionic liquid A in a 50 mL three-necked flask (i.e. ionic liquid [Hmim-PS] [HSO 4 ]). The temperature was raised to 85°C for reflux, and the reaction was stopped for 8 h. The reaction system is obviously layered, the lower layer is the catalyst, and the upper layer is the ester layer, which can be separated after cooling and pouring, and the recovered catalyst can be reused. Use 0.2 mol·L -1 The ester layer was titrated with KOH solution, and the esterification rate was calculated to be 92.6%. The upper ester layer was sequentially washed with 0.10mol·L -1 Wash with dilute aqueous sodium carbonate and distilled water, then rotary evaporate and dry to obtain diethyl maleate product.
Embodiment 2
[0031] as per figure 1 The structure shown builds the reaction apparatus.
[0032]Add 9.80 g (0.10 mol) of maleic anhydride, 18.43 g (0.40 mol) of absolute ethanol, 15 mL of cyclohexane (as a water carrier) and 3.22 g (0.02 mol) of acidic ionic liquid B into a 50 mL three-necked flask (i.e. ionic liquid [Hmim-PS][PTSA]). The temperature was raised to 85°C for reflux, and the reaction was stopped for 8 h. The reaction system is obviously layered, the lower layer is the catalyst, and the upper layer is the ester layer, which can be separated after cooling and pouring, and the recovered catalyst can be reused. Use 0.2 mol·L -1 The ester layer was titrated with KOH solution, and the esterification rate was calculated to be 85.6%. The upper ester layer was sequentially washed with 0.10mol·L -1 Wash with dilute aqueous sodium carbonate and distilled water, then rotary evaporate and dry to obtain diethyl maleate product.
Embodiment 3
[0034] as per figure 1 The structure shown builds the reaction apparatus.
[0035] Add 9.80 g (0.10 mol) of maleic anhydride, 18.43 g (0.40 mol) of absolute ethanol, 15 mL of cyclohexane (as water carrier) and 2.95 g (0.02 mol) of acidic ionic liquid C into a 50 mL three-necked flask (i.e. ionic liquid [Hmim-PS][CH 3 SO 3 ]). The temperature was raised to 85°C for reflux, and the reaction was stopped for 8 h. The reaction system is obviously layered, the lower layer is the catalyst, and the upper layer is the ester layer, which can be separated after cooling and pouring, and the recovered catalyst can be reused. Use 0.2 mol·L -1 The ester layer was titrated with KOH solution, and the esterification rate was calculated to be 79.4%. The upper ester layer was sequentially washed with 0.10mol·L -1 Wash with dilute aqueous sodium carbonate and distilled water, then rotary evaporate and dry to obtain diethyl maleate product.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com