Hypolipidemic medicament and preparation method thereof
A technology for lowering blood lipids and drugs, applied in the field of medicine, can solve problems such as stability to be improved, unfavorable commercial production, and reduced production efficiency, and achieve the effects of facilitating commercial production, benefiting production efficiency, and improving stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0019] Embodiment 1, prepare 1000 pieces of pitavastatin calcium tablets of 2mg
[0020] Pitavastatin calcium 2g, aluminum magnesium silicate 2.3g, montmorillonite 0.1g, lactose 95g, low-substituted hypromellose 18g, hypromellose 2g, magnesium stearate 0.6g, copovidone S630 0.8g and film coating premix (stomach-soluble type) 4g;
[0021] Preparation Process:
[0022] 1) Pass pitavastatin calcium, lactose, hypromellose, magnesium aluminum silicate, low-substituted hypromellose, and montmorillonite through a 60-mesh sieve, magnesium stearate and copovidone S630 through a 80-mesh sieve ,spare;
[0023] 2) Mix lactose, magnesium aluminum silicate, low-substituted hypromellose, montmorillonite, and hypromellose for 2 minutes; add pitavastatin calcium, mix for 8 minutes, and add magnesium stearate and 0.4 g of Copovidone S630, mixed for 5 minutes, and the uniformly mixed material was pressed into tablets;
[0024] 3) The film coating premix (stomach-soluble type) is formulated w...
Embodiment 2
[0026] Embodiment 2, prepare 1000 pieces of pitavastatin calcium tablets of 2mg
[0027] Pitavastatin calcium 2g, aluminum magnesium silicate 2.0g, montmorillonite 0.4g, lactose 95g, low-substituted hypromellose 18g, hypromellose 2g, magnesium stearate 0.6g, copovidone S630 0.8g and film coating premix (stomach-soluble type) 4g;
[0028] The preparation process is the same as in Example 1.
Embodiment 3
[0029] Embodiment 3, prepare 1000 pieces of pitavastatin calcium tablets of 2mg
[0030] Pitavastatin calcium 2g, aluminum magnesium silicate 2.1g, montmorillonite 0.3g, lactose 98g, low-substituted hypromellose 16g, hypromellose 3g, magnesium stearate 0.6g, copovidone S630 0.5g and film coating premix (stomach-soluble type) 4.3g;
[0031] The preparation process is the same as in Example 1.
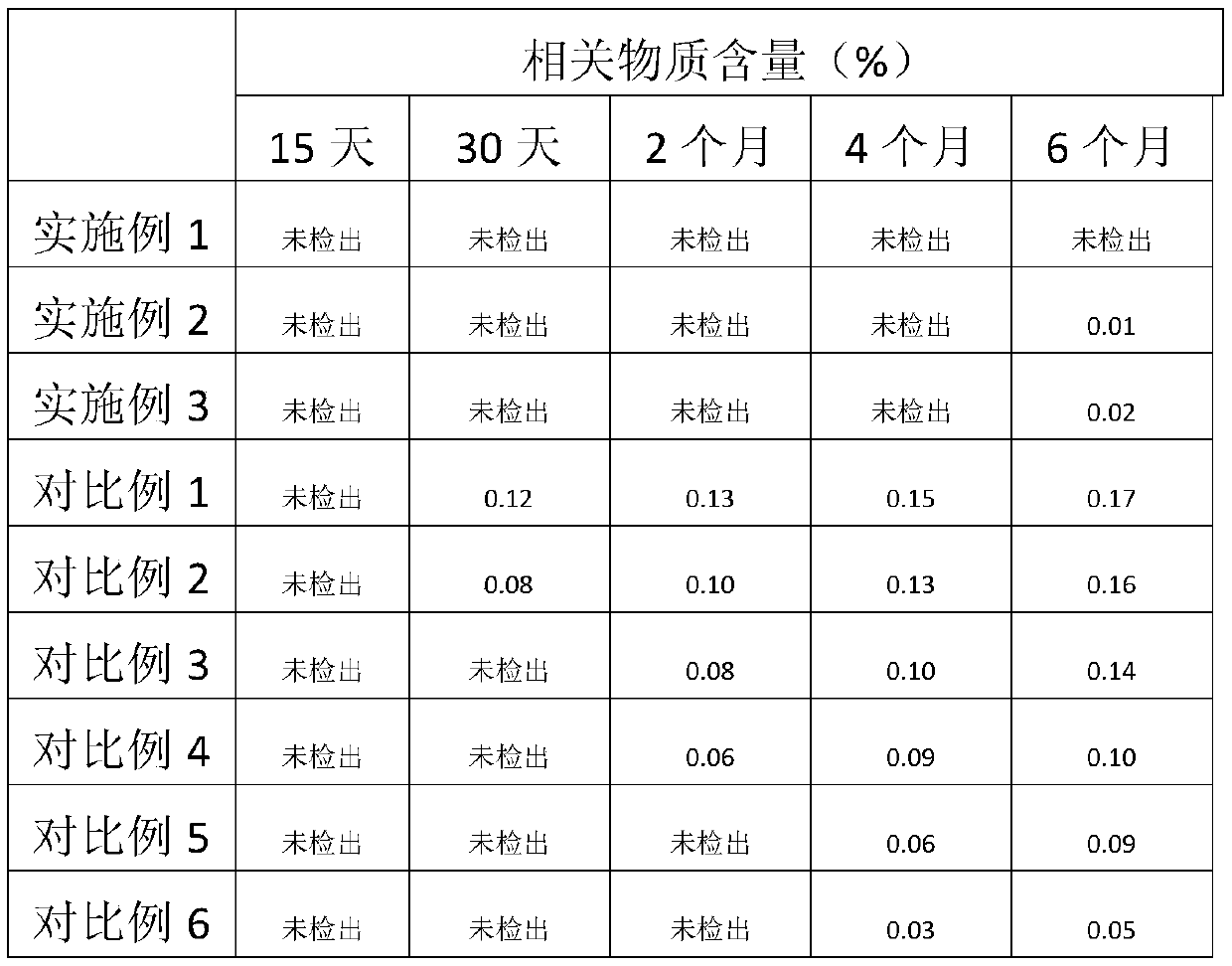
[0032] The content uniformity of the samples in Examples 1-3 was measured using the content uniformity detection method in the second appendix of the Pharmacopoeia of the 2015 edition. The content uniformity of each sample was A+2.2S less than or equal to 10.0, indicating that the sample had good content uniformity.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com