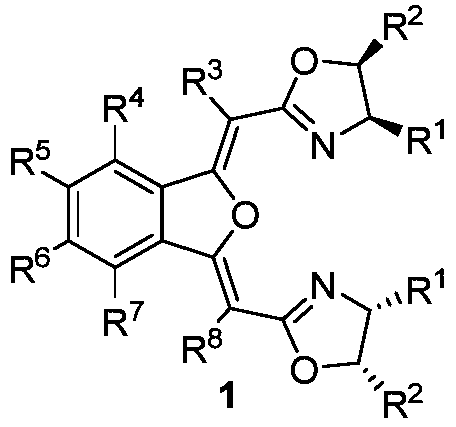
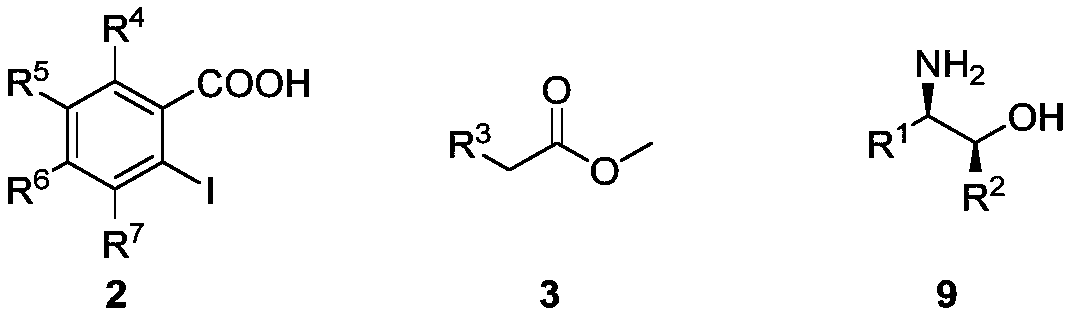
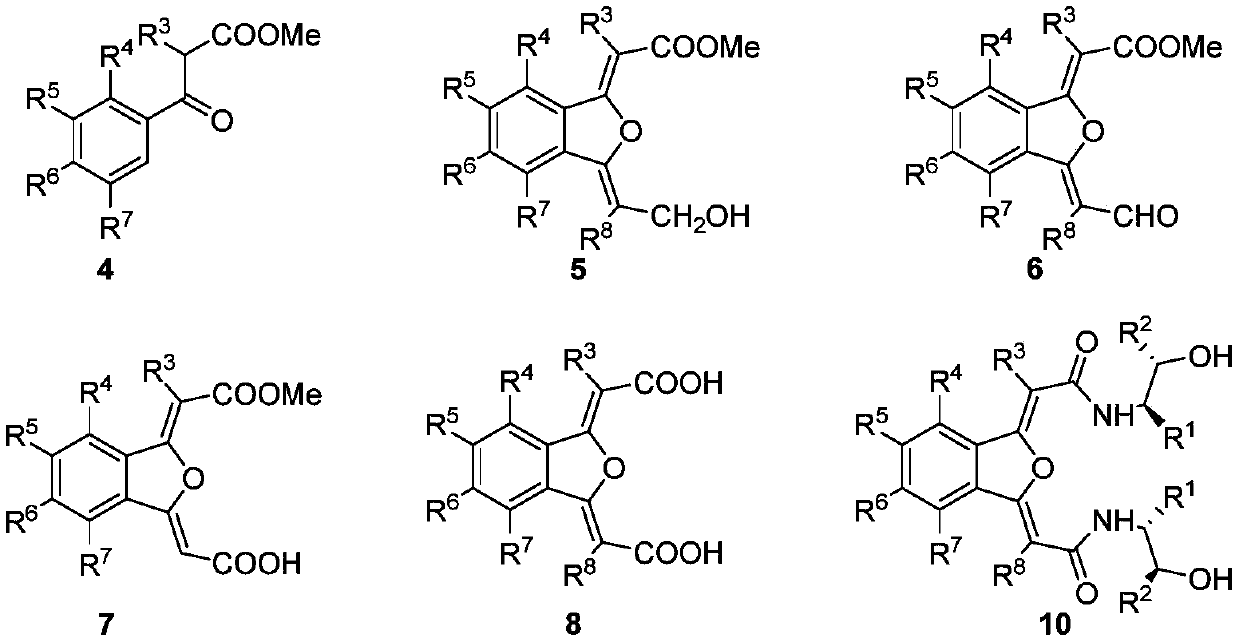
NON type chiral bisoxazoline ligand nd synthesis method and application thereof
A bisoxazoline and synthesis method technology, which is applied in the field of NON-type chiral bisoxazoline ligands and its synthesis, can solve the problems of insufficient synthesis method, harsh synthesis conditions, and few reaction types, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0059] Example 1: Synthesis of Intermediate 4.
[0060]
[0061] Under anhydrous and oxygen-free conditions, o-iodobenzoic acid compound 2 (4.96g, 20mmol, 1 equiv) was added to the reactor, nitrogen was replaced three times, then 80mL of dry dichloromethane was added to the reactor, and stirred at 0°C for 2 ~3 minutes, after the internal and external temperature stabilized, add two drops of N,N-dimethylformamide, and finally add oxalyl chloride (1.86mL, 22mmol, 1.1equiv) dropwise into the system using a constant pressure dropping funnel, half The drop was completed within hours, and finally the reactor was transferred to room temperature for reaction. After the reaction solution became transparent, TLC monitored it. After 6-7 hours, the reaction stopped, and was directly spin-dried to obtain o-iodobenzoyl chloride compounds. Under anhydrous and oxygen-free conditions, add methyl acetate 3 (6.4mL, 80mmol, 4equiv) into a 500mL reactor, then add 120mL of dry tetrahydrofuran or...
Embodiment 2
[0063] Example 2: Synthesis of Intermediate 5.
[0064]
[0065] Under anhydrous and oxygen-free conditions, the intermediate 4 (6.08g, 20mmol, 1equiv), cuprous iodide (381mg, 2mmol, 0.1equiv), and tetrakis (triphenylphosphine) palladium (1.156g, 1mmol, 0.05 equiv) were added to the reactor respectively, nitrogen was replaced three times, then propargyl alcohol (4.6mL, 80mmol, 4equiv) and triethylamine (8.3mL, 60mmol, 3equiv) were added to the reactor, and finally 200mL of dry tetrahydrofuran was added to the reactor , stirred at 50°C for 18h, TLC monitored R f=0.2 (ethyl acetate:petroleum ether=1:1, UV) the reaction was completed, and after the reaction device returned to room temperature, filter it directly with diatomaceous earth, and spin the filtrate to dryness. Intermediate 5 (3.99 g, 86%) was obtained by column chromatography separation with ethyl acetate:petroleum ether=1:5 or 1:1.
[0066] white solid. 1 H NMR (400MHz, CDCl 3 )δ7.62(d, J=3.8Hz, 1H), 7.60(d, J=3...
Embodiment 3
[0067] Example 3: Synthesis of Intermediate 6.
[0068]
[0069] under anhydrous and oxygen-free conditions. Intermediate 5 (4.64g, 20mmol, 1equiv) and Molecular sieves (6g) were added to the reactor, nitrogen was replaced three times, then 180mL of dry dichloromethane was added, and stirred at 0°C for 10 minutes. After the internal and external temperatures were stabilized, Dess-Martin reagent (8.48g, 20mmol, 1equiv) was slowly added to the reaction system. TLC monitoring reaction R f =0.5 (ethyl acetate:petroleum ether=1:1, UV), 5~10h after the reaction is over, return the reaction device to room temperature, filter directly with diatomaceous earth, slowly add sodium thiosulfate solution to the filtrate, stir for 30 minutes, Extract with ethyl acetate, combine the organic phases, add saturated sodium bicarbonate solution, wash twice with saturated sodium chloride solution, dry over anhydrous magnesium sulfate, and spin the filtrate to dryness. Using ethyl acetate:pet...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com