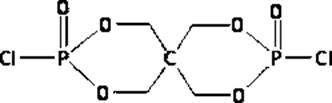Reactive intumescent flame retardant for polyurethane and synthesis method of reactive intumescent flame retardant
An intumescent flame retardant and a synthesis method technology, applied in the field of flame retardants and new intumescent flame retardants, can solve the problems of poor flame retardant effect, large amount of use, low content of phosphorus and nitrogen components, etc., and achieve easy control. , The effect of good carbon formation and high yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0028] The synthetic method of reactive expansion flame retardant for polyurethane comprises the following steps:
[0029] (1) Add 10.0 g of chlorinated spirocyclic phosphate and 50 mL of deionized water into a 250 mL reactor equipped with a stirring device and a cooling device, and stir vigorously for 5 minutes to make the chlorinated spirocyclic phosphate Evenly dispersed in water;
[0030] (2) Weigh 9.0 g of diethanolamine and dissolve it in 20 mL of deionized water, and add the aqueous solution of diethanolamine dropwise to the suspension obtained in step (1) at 3 °C under vigorous stirring;
[0031] (3) Measure 9.6 mL of triethylamine dropwise into the reaction system obtained in step (2). After the dropping is complete, raise the temperature to 55°C and react for 5 hours. yellow solution;
[0032] (4) Cool the solution obtained in step (3) to room temperature, filter, and concentrate under reduced pressure at 70°C until the volume of the solution does not change to obt...
Embodiment 2
[0038](1) Add 10.0 g of chlorinated spirocyclic phosphate and 50 mL of methanol into a 250 mL reactor equipped with a stirring device and a cooling device, and stir vigorously for 3 minutes to make the chlorinated spirocyclic phosphate evenly dissolved in the demethanol dispersion;
[0039] (2) Weigh 10.7 g of diethanolamine and dissolve it in 40 mL of deionized water, and add the aqueous solution of diethanolamine dropwise to the suspension obtained in step (1) at 0 °C under vigorous stirring;
[0040] (3) Measure 9.6 mL of triethylamine dropwise into the reaction system obtained in step (2). After the dropping is complete, raise the temperature to 60°C and react for 4 hours. yellow solution;
[0041] (4) Cool the solution obtained in step (3) to room temperature, filter, and concentrate under reduced pressure at 60°C until the volume of the solution does not change to obtain a yellow viscous liquid, which is washed 5 times with dichloromethane to purify product;
[0042] ...
Embodiment 3
[0044] (1) Add 10.0 g of chlorinated spirocyclic phosphate and 50 mL of methanol into a 250 mL reactor equipped with a stirring device and a cooling device, and stir vigorously for 4 minutes to make chlorinated spirocyclic phosphate in demethanol Evenly dispersed;
[0045] (2) Weigh 10.7 g of diethanolamine and dissolve it in 30 mL of methanol, and add the methanol solution of diethanolamine dropwise to the suspension obtained in step (1) at 5 °C under vigorous stirring;
[0046] (3) Measure 9.6 mL of triethylamine dropwise into the reaction system obtained in step (2). After the dropping is complete, raise the temperature to 50°C and react for 6 hours. yellow solution;
[0047] (4) Cool the solution obtained in step (3) to room temperature, filter, and concentrate under reduced pressure at 40°C to 1 / 3 of the volume of the original solution, then heat up to 60°C and continue to concentrate under reduced pressure until the volume of the solution does not change, to obtain Yel...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com