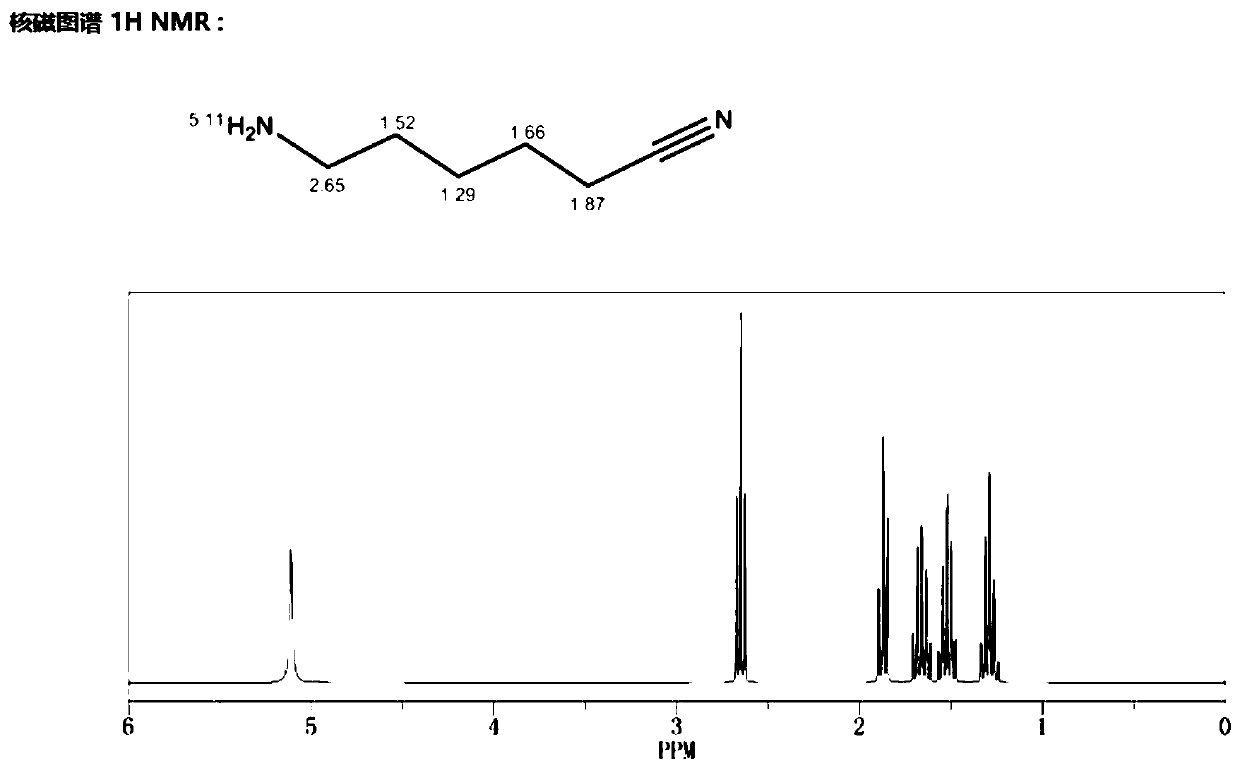
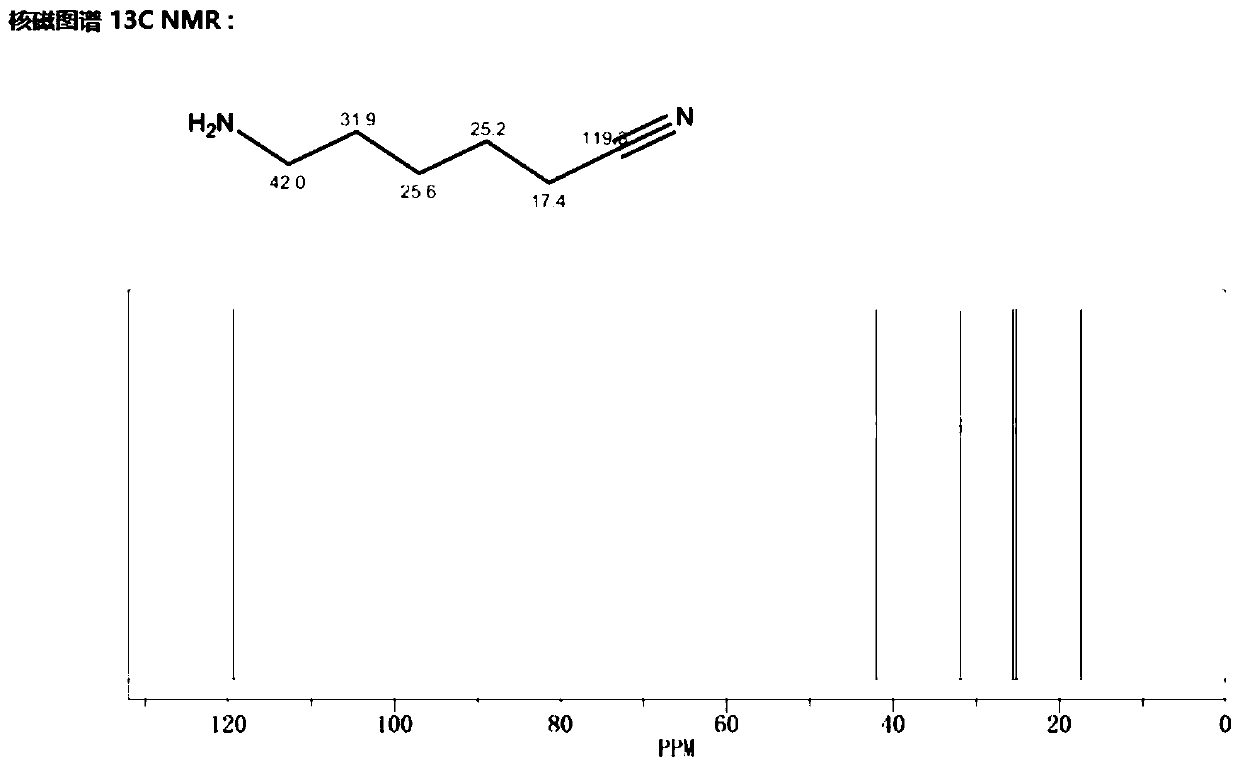
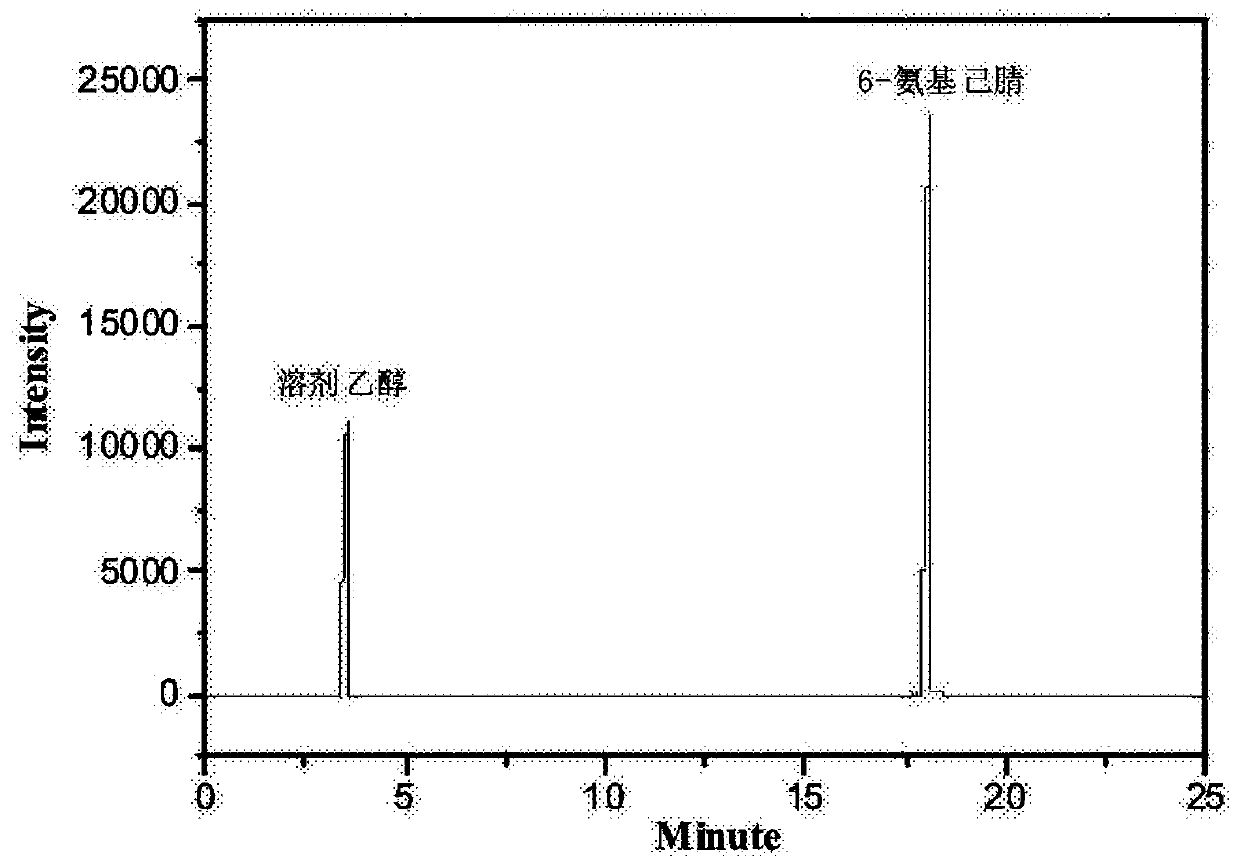
Method for preparing 6-aminocapronitrile product
A technology of aminocapronitrile and aminocaproate, applied in the field of preparation of aminonitrile, can solve the problems of unsuitability for large-scale industrialization, harsh reaction conditions, low reaction conversion rate, etc., and achieves saving purification unit consumption, less side reactions, high purity effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0034] Mix 10g of caprolactam and 100g of formic acid aqueous solution evenly, then add them into reaction kettle A and stir and heat at a stirring speed of 1000RPM. When the temperature rises to 110°C, the reflux reaction starts. After reflux for 2 hours, the reaction liquid is discharged to rectification kettle C. Distill to remove low boilers, transfer the rectification still liquid to reactor B, and use methanol-toluene (the volume ratio of methanol to toluene is 1:0.8) for recrystallization (stirring rate is 180 r / min, cooling rate is 2.5°C / min) to get 6-aminocaproic acid salt solid, drain the liquid in the kettle, add 30g of ethylene glycol, 25g of ammonium oxalate and 1g of phosphorus oxychloride into the kettle, mix evenly, stir and heat, the stirring speed is 1000RPM, and reflux reaction at 105°C Discharge after 3h. After the reaction, the reaction liquid was rectified under reduced pressure, separated and purified to obtain 6-aminocapronitrile with a purity of 98.3%...
Embodiment 2
[0037] Mix 10g of caprolactam and 150g of formic acid aqueous solution evenly, then add them into reaction kettle A, stir and heat, the stirring speed is 1100RPM, when the temperature rises to 112°C, the reflux reaction starts, and after reflux for 1.5h, the reaction liquid is discharged to rectification kettle C , to remove low boilers by distillation, transfer the rectification still liquid to reactor B, and recrystallize with ethylene glycol-toluene (the volume ratio of ethylene glycol and toluene is 1:0.5) (stirring speed is 150 r / min, cooling The rate is 2°C / min) to get 6-aminocaproic acid salt solid, drain the liquid in the kettle, add 40g of ethylene glycol, 35g of ammonium oxalate and 0.5g of ethyl phosphate into the kettle, stir and heat after mixing evenly, the stirring speed is 1100RPM , After reflux reaction at 150°C for 5h, the material was discharged. After the reaction, the reaction solution was rectified under reduced pressure, separated and purified to obtain ...
Embodiment 3
[0040]Mix 11g of caprolactam and 155g of acetic acid aqueous solution evenly, then add them into reaction kettle A, stir and heat, the stirring speed is 1200RPM, when the temperature rises to 110°C, the reflux reaction starts, and after reflux for 3 hours, the reaction liquid is discharged to rectification kettle C, Distill to remove low boilers, transfer the rectification still liquid to reactor B, and use methanol-p-xylene (the volume ratio of methanol and p-xylene is 1:0.8) for recrystallization (stirring rate is 190 r / min, cooling rate 3.5°C / min) to get 6-aminocaproic acid salt solid, drain the liquid in the kettle, add 40g of ethylene glycol, 35g of formamide and 0.6g of ethyl phosphate into the kettle, stir and heat after mixing evenly, the stirring speed is 1200RPM, After reflux reaction at 130°C for 2 hours, the material was discharged. After the reaction, the reaction solution was rectified under reduced pressure, separated and purified to obtain 6-aminocapronitrile w...
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com