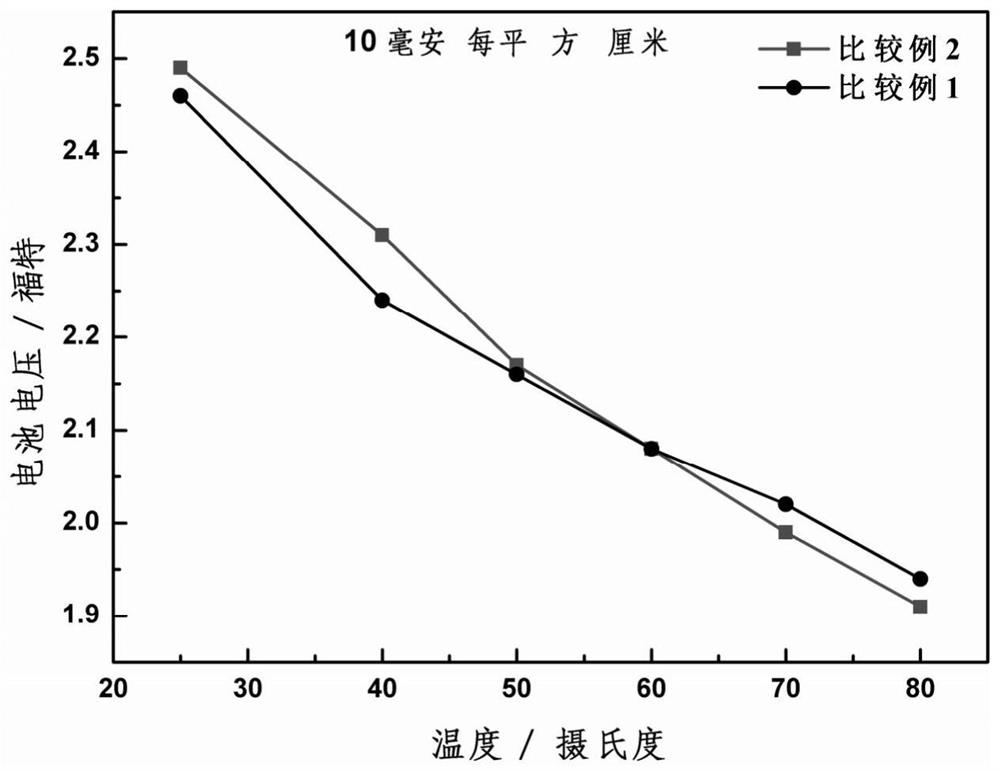
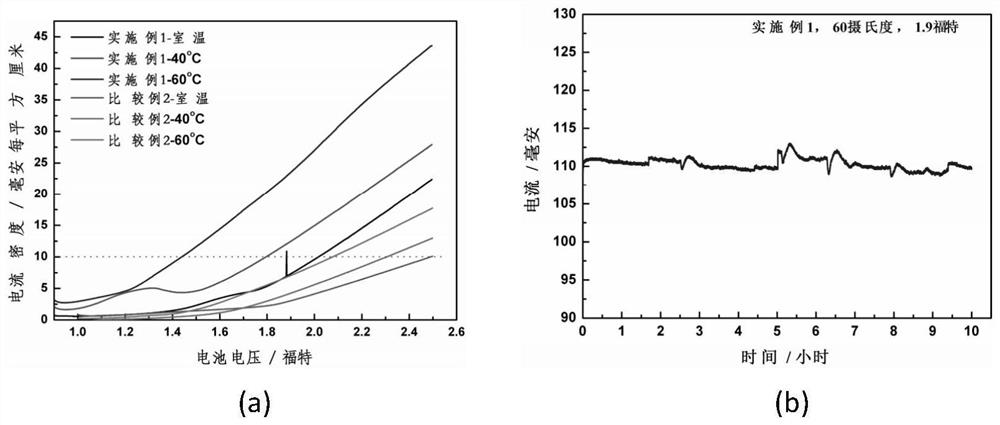
An alkaline water electrolysis full battery
A water electrolysis cell, full battery technology, applied in the direction of electrolysis components, electrolysis process, electrodes, etc., can solve the problem of difficult to achieve high-efficiency catalysis, and achieve the goal of promoting electron transfer and material transfer, stable performance, and reducing initial potential. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0047] 1. Electrode material pretreatment: nickel content ≥ 99.5%, thickness 100 microns, area 10 cm 2 The nickel sheet is used as the base material, firstly through 800# and 1200# # Polish the surface with water abrasive paper until the surface is smooth, then soak it in acetone at room temperature for 15 minutes to remove the surface grease; finally soak it in 0.5M hydrochloric acid aqueous solution for 20 minutes to remove the surface scale, and then rinse with a large amount of deionized water To neutral, dry with high-purity Ar gas;
[0048] 2. Electroplating solution preparation: to analyze pure grade CoSO 4 ·7H 2 O and CoCl 6H 2 O as Co salt, H 3 BO 3 As a pH stabilizer, prepare 230ml electroplating solution with ultrapure water with a resistivity of 18.2MΩ, and control the CoSO in the plating solution 4 ·7H 2 O, CoCl 6H 2 O and H 3 BO 3 The concentrations are 0.5M, 0.2M and 0.1M, respectively.
[0049] 3. Electrodeposition of Co particles: First, under the s...
Embodiment 2
[0055] 1. Electrode material pretreatment: nickel content ≥ 99.5%, thickness 50 microns, area 10 cm 2 The nickel sheet is used as the base material, firstly through 800# and 1200# # Polish the surface with water abrasive paper until the surface is smooth, then soak it in acetone at room temperature for 15 minutes to remove the surface grease; finally soak it in 0.5M hydrochloric acid aqueous solution for 30 minutes to remove the surface scale, and then rinse with a large amount of deionized water To neutral, dry with high-purity Ar gas;
[0056] 2. Electroplating solution preparation: to analyze pure grade CoSO 4 ·7H 2 O and CoCl 6H 2 O as Co salt, H 3 BO 3 As a pH stabilizer, prepare 230ml electroplating solution with ultrapure water with a resistivity of 18.2MΩ, and control the CoSO in the plating solution 4 ·7H 2 O, CoCl 6H 2 O and H 3 BO 3 The concentrations are 0.3M, 0.1M and 0.5M, respectively.
[0057] 3. Electrodeposition of Co particles: First, under the st...
Embodiment 3
[0061] 1. Electrode material pretreatment: nickel content ≥ 99.5%, thickness 1mm, area 10cm 2 The nickel sheet is used as the base material, firstly through 800# and 1200# #Polish the surface with water abrasive paper until the surface is smooth, then soak it in acetone at room temperature for 30 minutes to remove surface grease; finally soak it in 0.5M hydrochloric acid aqueous solution for 30 minutes to remove surface oxide skin, and then rinse with a large amount of deionized water To neutral, dry with high-purity Ar gas;
[0062] 2. Electroplating solution preparation: to analyze pure grade CoSO 4 ·7H 2 O and CoCl 6H 2 O as Co salt, H 3 BO 3 As a pH stabilizer, prepare 230ml electroplating solution with ultrapure water with a resistivity of 18.2MΩ, and control the CoSO in the plating solution 4 ·7H 2 O, CoCl 6H 2 O and H 3 BO 3 The concentrations are 1.0M, 0.5M and 0.3M, respectively.
[0063] 3. Electrodeposition of Co particles: First, under the stirring condi...
PUM
| Property | Measurement | Unit |
|---|---|---|
| thickness | aaaaa | aaaaa |
| width | aaaaa | aaaaa |
| thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com