Cellulose electron transport polymer as well as preparation method and application thereof
A technology for electron transport and polymers, applied in circuits, photovoltaic power generation, electrical components, etc., can solve the problems of cellulose and its derivatives that have not been reported
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
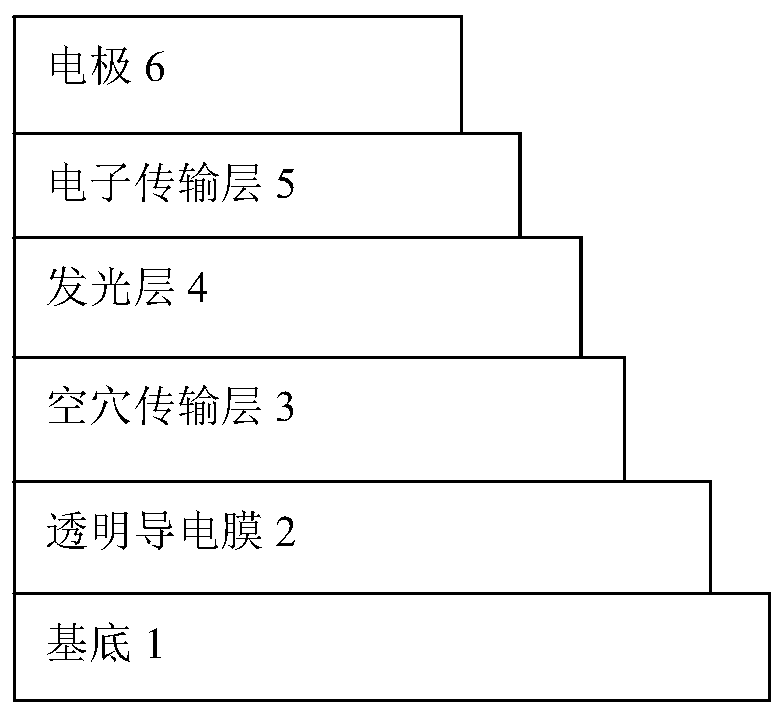
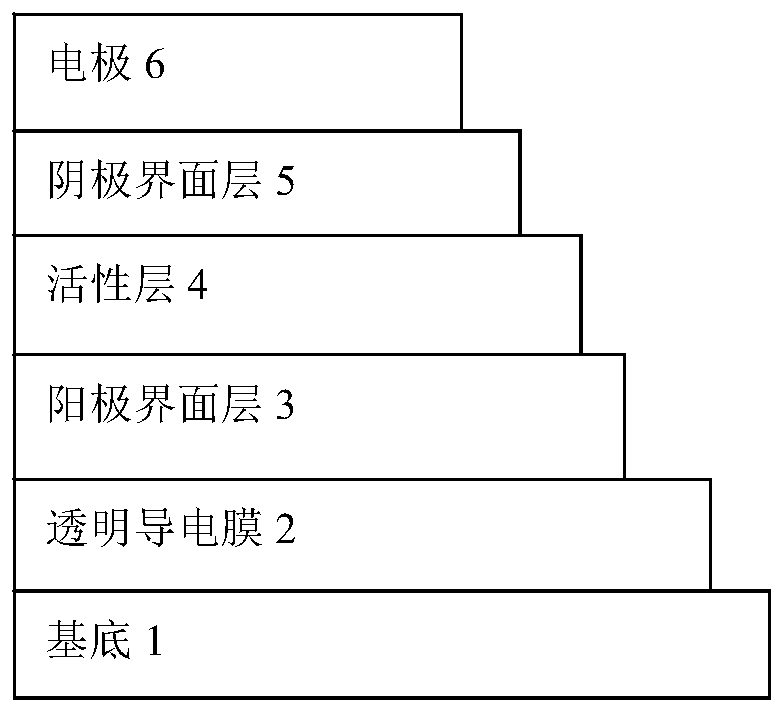
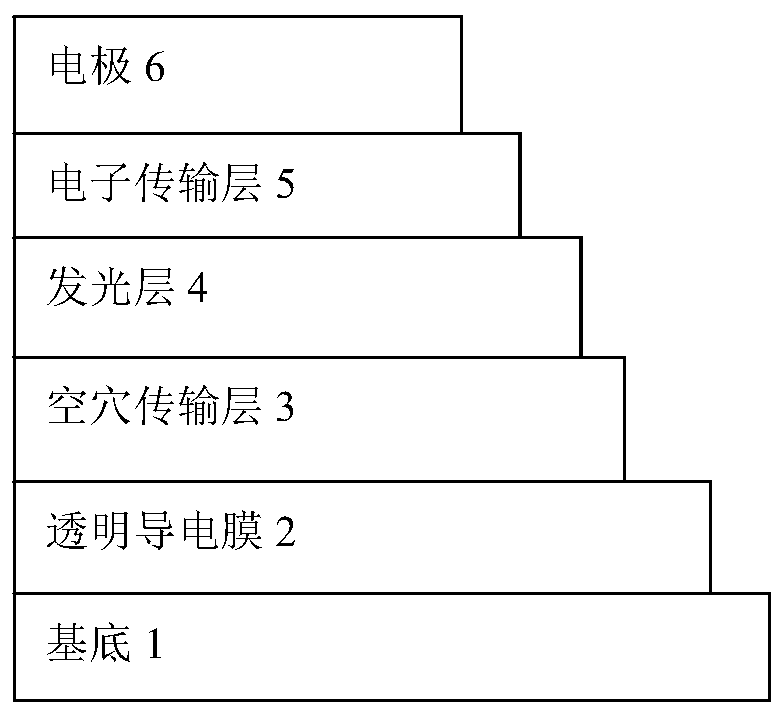
Image
Examples
Embodiment 1
[0091] Synthesis of embodiment 1 formula Ⅰ-1 compound
[0092] In this embodiment, the structural formula of the compound of formula I-1 is:
[0093]
[0094] The preparation method of above-mentioned formula I-1 compound comprises the steps:
[0095] Step 1: Synthesis of intermediate formula, bis[4-(dimethylamino)phenyl]-4-hydroxyphenylphosphine
[0096] 1 part of starter Dissolve 2 parts of p-iodophenyldimethylamine in 250 mL of tetrahydrofuran solution, protect with argon, cool down to -75°C, stir at this temperature for 60 minutes, add 4 parts of tert-butyllithium n-hexane solution dropwise In the reaction system, the reaction system was then warmed up to room temperature and stirred for 60 minutes;
[0097] Then put the reaction system again at -75°C and stir for 30 minutes, add 1 part of phosphorus trichloride n-pentane solution dropwise into the reaction system, heat up to room temperature, and react for 12 hours;
Embodiment 2
[0107] Synthesis of embodiment 2 formula Ⅰ-2 compound
[0108] In the present embodiment, the structural formula of the compound of formula I-2 is:
[0109]
[0110] Step 1: Synthesis of intermediate bis[4-(dimethylamino)phenyl]-4-hydroxyphenylphosphine
[0111] 2 parts of the starter Dissolve 4 parts of p-iodophenyldimethylamine in 250 mL of tetrahydrofuran solution, protect with argon, cool down to -75°C, stir at this temperature for 120 minutes, add 8 parts of tert-butyllithium n-hexane solution dropwise In the reaction system, the reaction system was then warmed up to room temperature and stirred for 1200 minutes;
[0112] Then put the reaction system again at -75°C and stir for 30 minutes, add 2 parts of phosphorus trichloride n-pentane solution dropwise into the reaction system, heat up to room temperature, and react for 18 hours;
[0113] Then add 6 parts of hydrogen peroxide to the above solution, stir at room temperature for 120 minutes, stop the reaction, re...
Embodiment 3
[0122] Synthesis of embodiment 3 formula Ⅱ-1 compound
[0123] In the present embodiment, the structural formula of the compound of formula II-1 is:
[0124]
[0125] The preparation method of above-mentioned formula II-1 compound comprises the steps:
[0126] Step 1: Synthesis of intermediate 4-((4-dimethylamino)phenyl)sulfone)phenol
[0127] 1 part of starter Dissolve 1 part of p-iodophenyldimethylamine and 0.1 part of cesium carbonate in 250 mL of tetrahydrofuran solution, under argon protection, stir and heat up to reflux, and react for 12 hours; then add 2 parts of hydrogen peroxide to the above solution, and stir at room temperature After 60 minutes, the reaction was stopped, and after the solvent was removed, the intermediate 4-((4-dimethylamino)phenyl)sulfone)phenol was obtained as a white powder through column separation and purification, with a yield of 75%.
[0128] Mass spectrum [M] + : 275.08; 1 H NMR (CDCl 3 ): δ (ppm) = 7.82 (d, J = 8.82Hz, 2H), 7.32...
PUM
| Property | Measurement | Unit |
|---|---|---|
| thickness | aaaaa | aaaaa |
| thickness | aaaaa | aaaaa |
| thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com