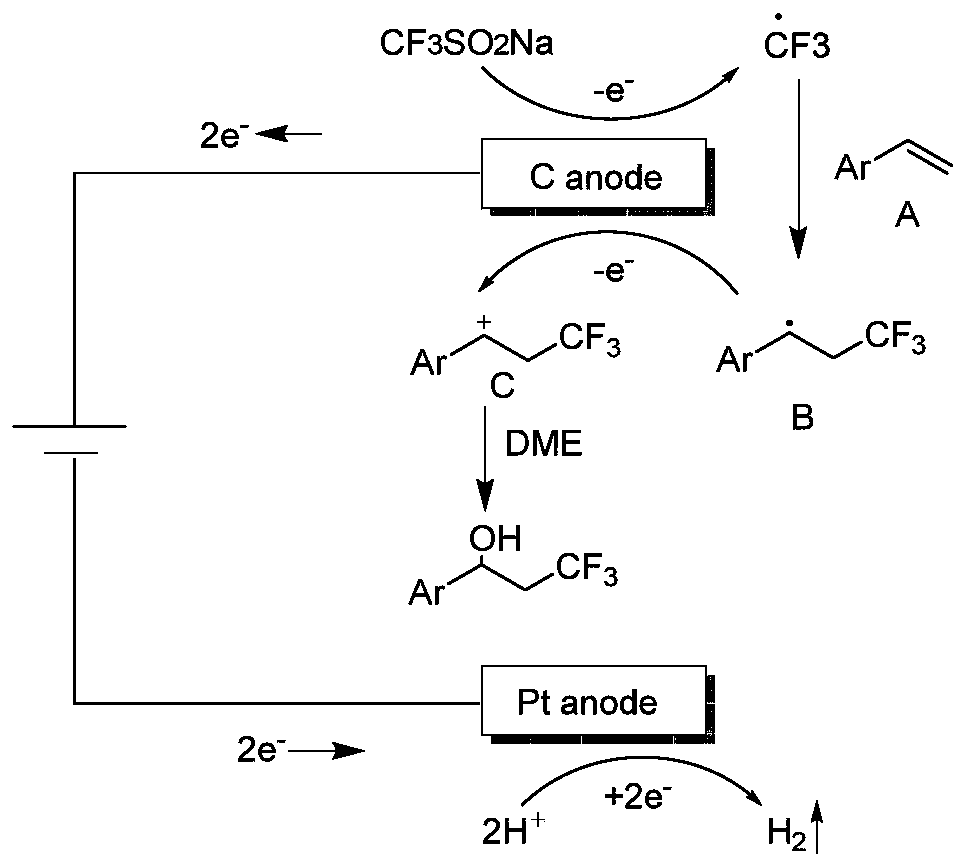
Synthesis method of beta-trifluoromethyl substituted alcohol organic molecule
A technology of trifluoromethyl and synthetic methods, which is applied in the field of synthesis of alcohol organic molecules, can solve problems such as limited synthetic routes and lack of research on trifluoromethyl hydroxylation, and achieves reduced synthesis costs, high yields, and low reaction rates. The effect of simple system components
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0029] Take a washed and dried pressure-resistant tube, add a magnet, then weigh 0.2mmol p-tert-butylstyrene, 0.4mmol sodium trifluoromethanesulfinate, 0.1M lithium perchlorate, and start stirring. While stirring, 4 ml of ethylene glycol dimethyl ether was added, followed by the slow addition of 0.4 ml of trifluoroacetic acid. Then insert the counter electrode, connect and turn on the power supply, set the constant current mode, the current size is 15mA, and react at room temperature for 6 hours. After the reaction was completed, the reaction solution was poured into saturated brine, extracted three times with dichloromethane (30ml×3), dried over anhydrous sodium sulfate, filtered, rotary evaporated, loaded, and subjected to column chromatography to obtain the final product. Trifluorotoluene was used as a calibration substance, and its concentration was calibrated by fluorine spectrum, and the yield was about 90%.
Embodiment 2
[0031] Take a washed and dried pressure-resistant tube, add a magnet, then weigh 0.2mmol of p-methylstyrene, 0.4mmol of sodium trifluoromethanesulfinate, and 0.1M lithium perchlorate in turn, start stirring, and 4 ml of ethylene glycol dimethyl ether was added with stirring, followed by the slow addition of 0.4 ml of trifluoroacetic acid. Then insert the counter electrode, connect and turn on the power supply, set the constant current mode, the current size is 15mA, and react at room temperature for 6 hours. After the reaction was completed, the reaction solution was poured into saturated brine, extracted three times with dichloromethane (30ml×3), dried over anhydrous sodium sulfate, filtered, rotary evaporated, loaded, and subjected to column chromatography to obtain the final product. Trifluorotoluene was used as a calibration substance, and its concentration was calibrated by fluorine spectrum, and the yield was about 92%.
Embodiment 3
[0038]Take a washed and dried pressure-resistant tube, add a magnet, then weigh 0.2mmol p-chlorostyrene, 0.4mmol sodium trifluoromethanesulfinate, 0.1M lithium perchlorate, start stirring, and stir While adding 4ml of ethylene glycol dimethyl ether, then slowly added 0.4ml of trifluoroacetic acid. Then insert the counter electrode, connect and turn on the power supply, set the constant current mode, the current size is 15mA, and react at room temperature for 6 hours. After the reaction was completed, the reaction solution was poured into saturated brine, extracted three times with dichloromethane (30ml×3), dried over anhydrous sodium sulfate, filtered, rotary evaporated, loaded, and subjected to column chromatography to obtain the final product. Trifluorotoluene was used as a calibration substance, and its concentration was calibrated by fluorine spectrum, and the yield was about 94%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com