A pharmaceutical composition and pharmaceutical preparation for promoting blood circulation, removing blood stasis, dredging channels and relieving pain
A pharmaceutical preparation, a technique of promoting blood circulation and removing blood stasis, which is applied in the field of traditional Chinese medicine, can solve the problems of unclear active ingredients, short drug effect duration, and inaccurate drug dosage, so as to achieve clear drug effect material basis, drug effect time, etc. Long-lasting, clear effect of medicinal ingredients
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
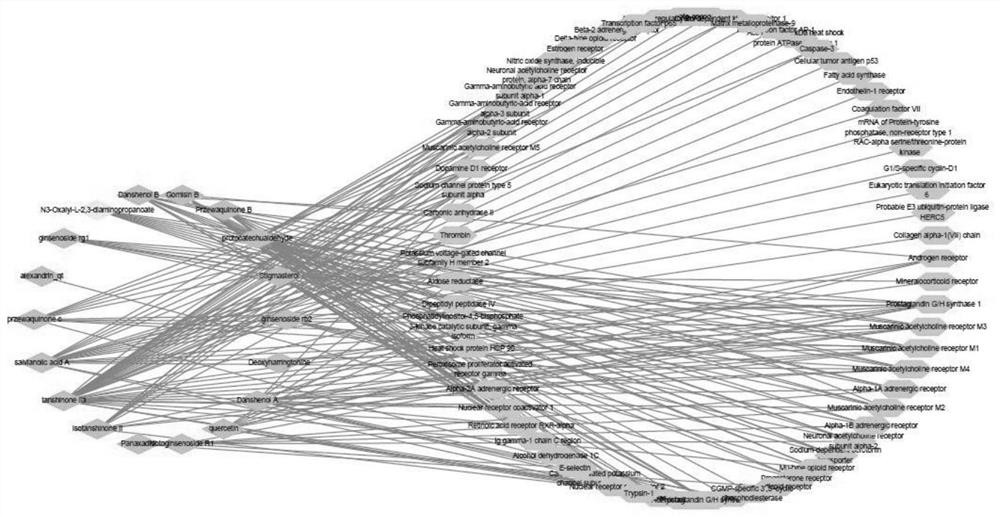
Image
Examples
Embodiment 1
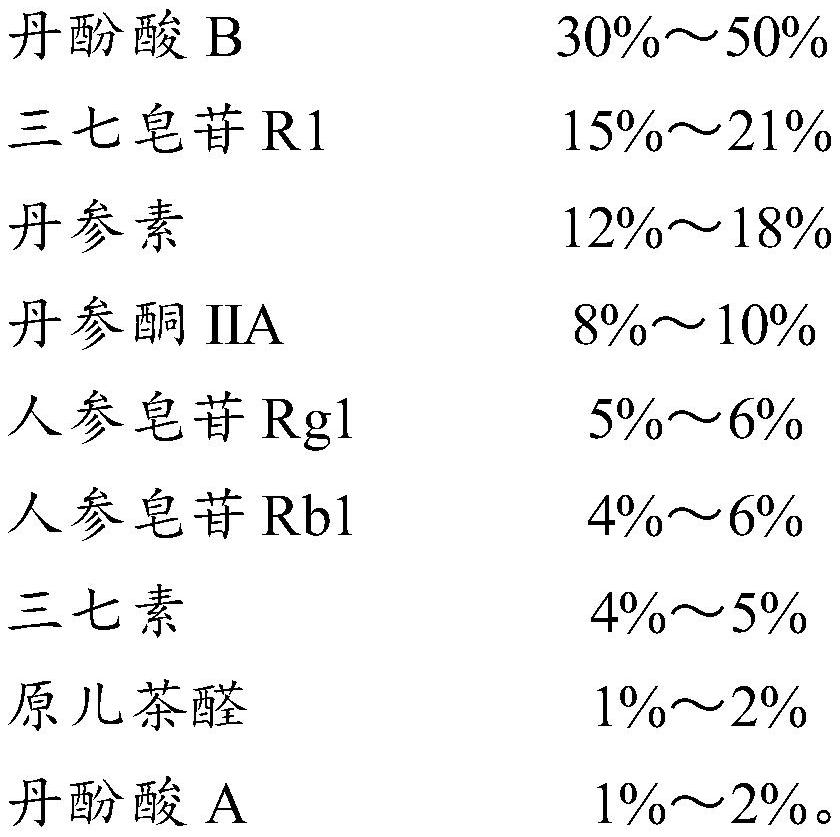
[0051] Take salvianolic acid B 20g, notoginsenoside R1 9g, danshensu 7.5g, tanshinone IIA 4.5g, ginsenoside Rg12.75g, ginsenoside Rb1 2.5g, notoginseng 2.25g, protocatechualdehyde 0.75g, salvianol Acid A 0.75g was mixed uniformly to make 50g.
Embodiment 2
[0053] Take 15g of salvianolic acid B, 10.5g of notoginseng saponin R1, 9g of danshensu, 5g of tanshinone IIA, 3g of ginsenoside Rg1, 3g of ginsenoside Rb1, 2.5g of notoginseng, 1g of protocatechualdehyde, 1g of salvianolic acid A and mix well , made into 50g.
Embodiment 3
[0055] Take salvianolic acid B 25g, notoginsenoside R1 7.5g, danshensu 6g, tanshinone IIA 4g, ginsenoside Rg12.5g, ginsenoside Rb1 2g, notoginseng 2g, protocatechualdehyde 0.5g, salvianolic acid A 0.5g g and mix well to make 50g.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com