Preparation method of low-valence halide of variable-valence metal
A technology of variable valence metals and halides, applied in the field of material science, can solve the problems of low concentration and large dosage, and achieve the effects of simple operation, low equipment requirements and strong practicability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0048] The preparation process of the electrolyte is as follows: First, the raw material reagents are transferred to a glove box with water and oxygen content less than 10ppm for storage; high-purity anhydrous AlI is prepared according to the molar ratio of 1:1 3 -KI (purity greater than 99%), after mixing evenly, put it in a corundum crucible, heat up to 300 ° C; then use a two-electrode system for electrolysis, the anode and the anode are both made of aluminum rods, and after constant current electrolysis for 10 hours, use 200 mesh molybdenum Net filter to remove suspended impurities, that is.
[0049] The preparation method of other molten salt electrolytes is the same as above.
Embodiment 1
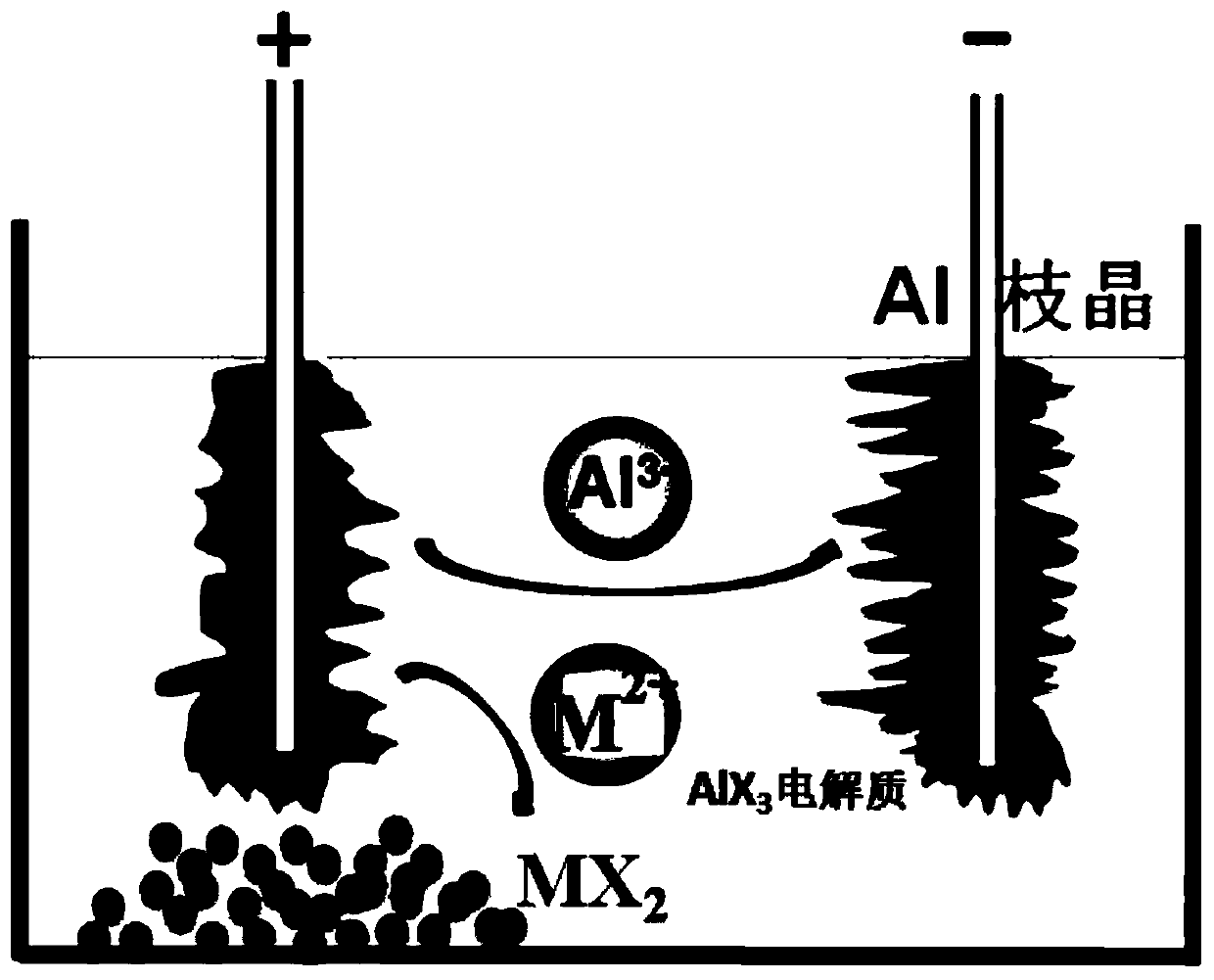
[0051] 50g AlI 3 -KI molten salt (AlI3 and KI molar ratio is 1:1) is placed in the alumina crucible, and the temperature control is 280 ℃; figure 1 It is a schematic diagram of electrolysis, using high-purity metal samarium (purity not less than 99%) as the anode, aluminum wire as the cathode (purity not less than 99.999%); the control cell voltage is 2.0V, electrolysis for 2 hours, and the electrolysis current is maintained at about 60mA About; After the electrolysis, filter with a 200-mesh metal molybdenum filter to obtain a blue precipitate; vacuum distill the blue precipitate at 500°C for 1 hour to obtain 0.766g such as figure 2 The black-green samarium dichloride solid is shown.
[0052] Such as image 3 Shown is a photo of the prepared samarium dichloride dissolved in tetrahydrofuran, which is dark blue.
[0053] Simultaneously, adopt weighing method, compare the weight loss of anode and the quality that obtains product, record the recovery rate of samarium diiodide ...
Embodiment 2
[0055] This embodiment provides a kind of method for preparing samarium diiodide, and the difference with embodiment 1 is only, change high-purity metal samarium into samarium aluminum alloy, electrolytic current has increased 3 times, and this method prepares samarium diiodide The recovery rate was 89%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com