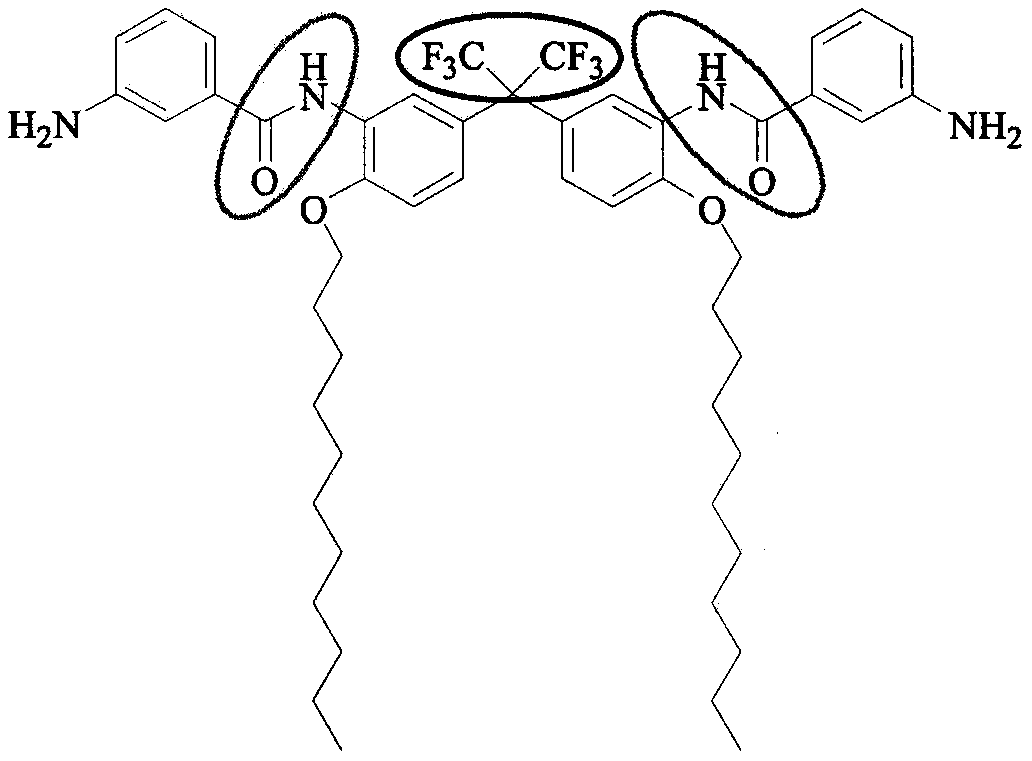
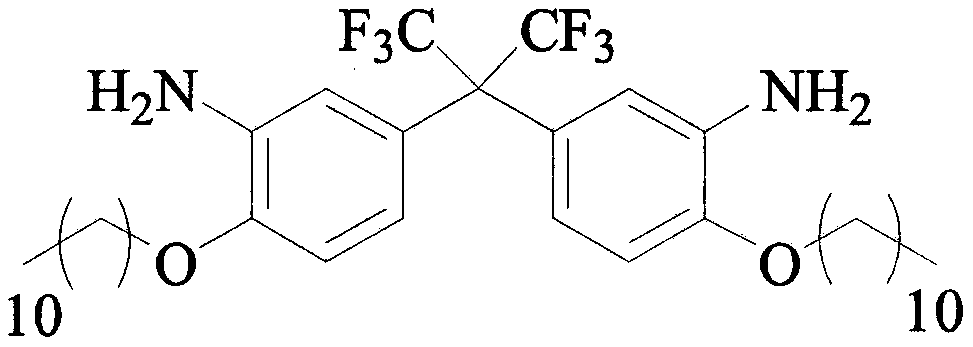
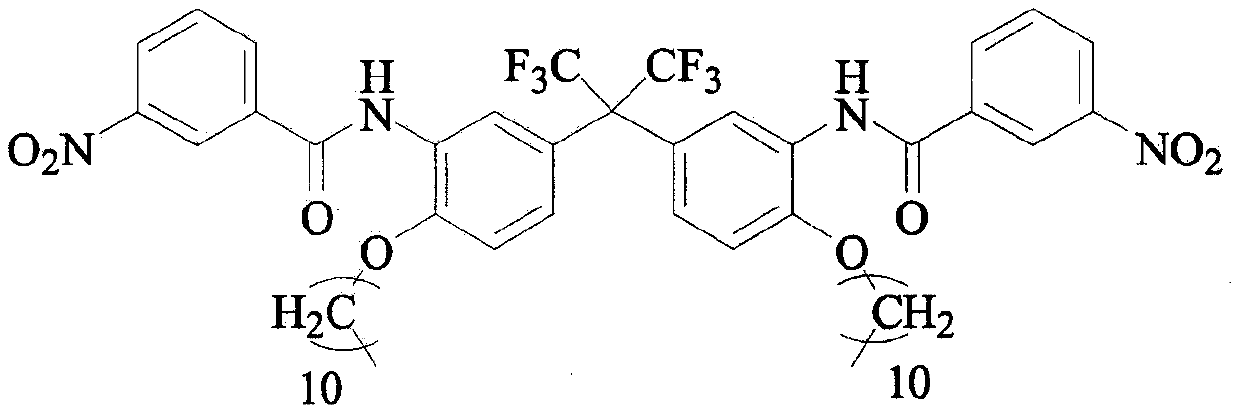
Preparation method of C11 side chain substituted fluorine-containing diamine monomer
A technology of fluorine-containing diamine, C11-FBDA, which is applied in the field of fine chemical industry and polymer chemistry, and the preparation of polyimide polymers. Reducing problems such as difficulty, achieving high synthesis yield, improving light transmittance, reducing order and symmetry
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0027] Step 1: Add 1.2L DMAC, 1000g 2,2-bis(3-amino-4-sodium phenolate) hexafluoropropane and 1142g bromo C11 alkane to a 10L four-necked reaction flask in sequence, start stirring and heating, and maintain React at 80°C for 2-3 hours. After the reaction of the raw materials is completed, turn off the heating, pour out the reaction liquid, and filter while it is hot. The upper layer of filter cake is the by-product sodium bromide, which is collected and stored. The filtrate is naturally cooled to room temperature, and then added to 2.4 Quenched in L water, a large amount of solids will appear at this time, filter with suction, wash the filter cake with 3.5L of water, and dry the filter cake in vacuum to obtain 1480g of pure C11-FN.
[0028] The second step: add 1480g C11-FN and 550mL NMP to the 10L four-necked reaction flask, start stirring, then slowly dropwise add the mixed solution of 812g m-nitrobenzoyl chloride and 550mL NMP to it, and control the concentration of the reac...
Embodiment 2
[0031] Step 1: Add 24.3L DMAC, 1000g 2,2-bis(3-amino-4-sodium phenolate) hexafluoropropane and 5709g bromo C11 alkane to the 30L double-layer glass reactor in sequence, start stirring and heating, Maintain the reaction at 166°C for 2-3 hours. After the reaction of the raw materials is completed, turn off the heating, release the reaction liquid, and filter while it is hot. The upper layer of filter cake is the by-product sodium bromide, which is collected and stored. The filtrate is naturally cooled to room temperature, and then added to 48.6 Quenched in L water, a large amount of solids will appear at this time, filter with suction, wash the filter cake with 3.5L of water, and dry the filter cake in vacuum to obtain 1563g of pure C11-FN.
[0032] Step 2: Add 1563g C11-FN and 11.5L NMP to the 30L double-layer glass reactor, start stirring and jacket cooling, when the temperature of the reaction solution drops to 0°C, slowly add 4286g m-nitrobenzene dropwise For the mixed solut...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com