Synthesis method of tulathromycin and synthesis method of tulathromycin phosphate
A technology of terramycin and a synthesis method, applied in the field of compound preparation, can solve problems such as unfavorable operation, unfavorable operation and cost saving, and achieve the effects of cost saving, low equipment requirements and mild reaction conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
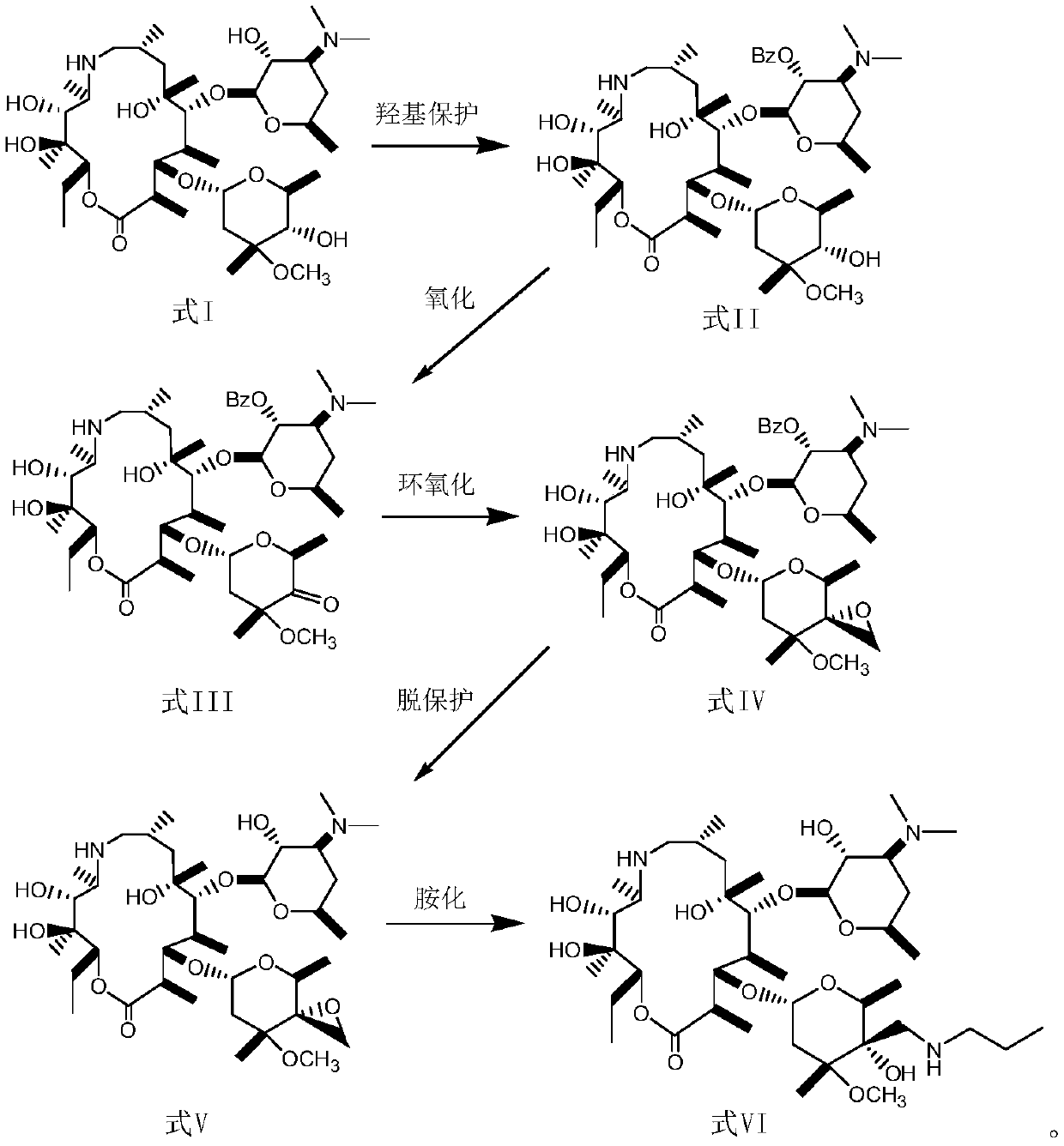
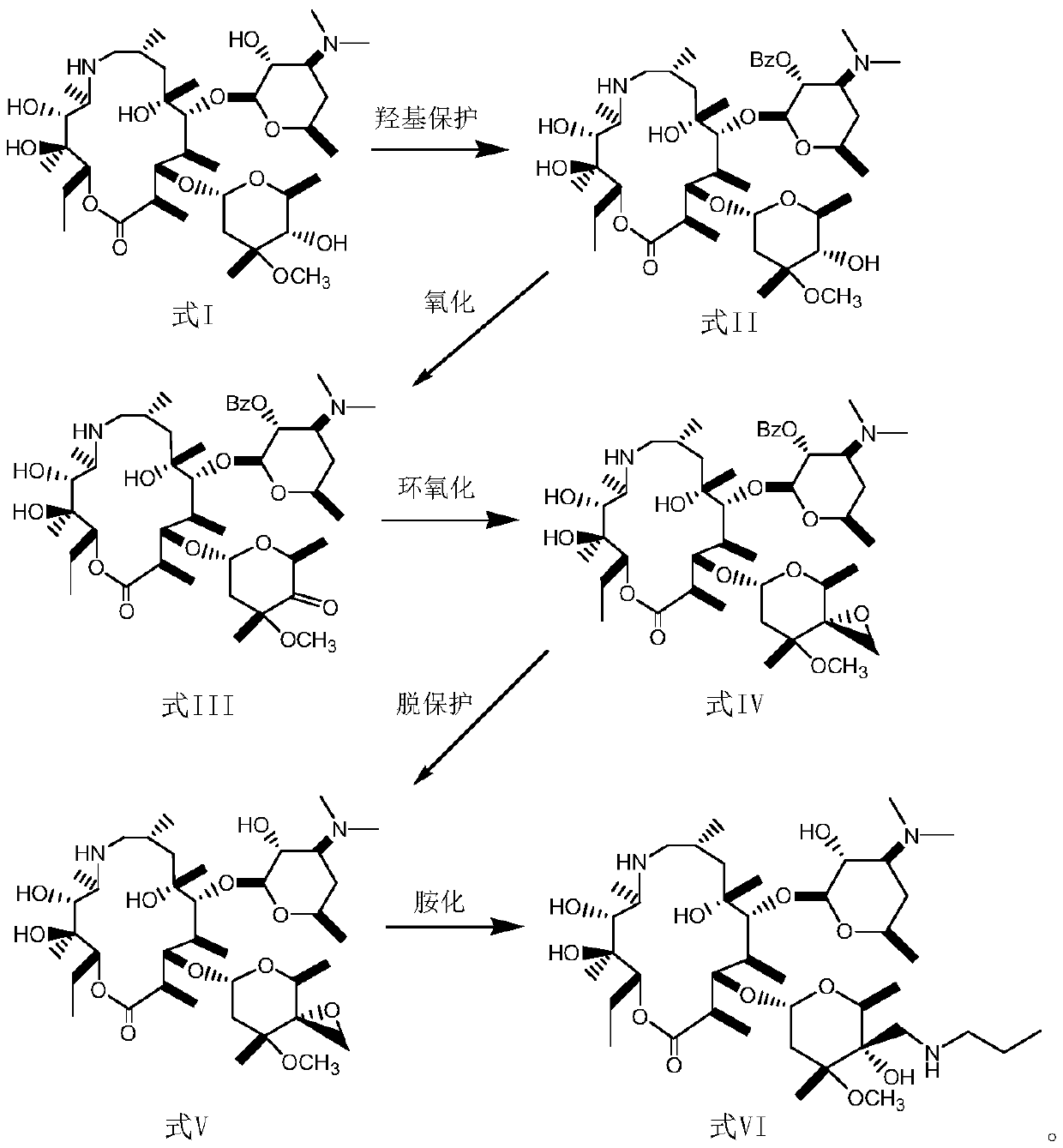
[0034] Hydroxyl protection steps:
[0035] In a low-temperature reaction tank, add 20 g of demethylazithromycin shown in formula I to a three-necked flask at a temperature of -10-0°C, dissolve it in 320 mL of dichloromethane, then cool to 3°C, stir, and slowly 4.7g of CBZ-Cl was added dropwise, and after 10 minutes, 2.7g of triethylamine was slowly added dropwise. The pH of the reaction solution was slightly alkaline, and stirred at 3°C for 2h. The end point of the reaction was monitored by thin-layer chromatography, and the reaction was completed. Add 150mL of distilled water, adjust the pH to 2 with 3N HCl, separate the liquids, extract the organic phase with an acidic water phase, combine the water phases, extract the water phase with petroleum ether and dichloromethane twice, add 150 mL of dichloromethane to the water phase Chloromethane, adjust pH=9 with 5N NAOH, separate the liquids, extract the aqueous phase with 50 mL of dichloromethane, combine the organic phases, d...
Embodiment 2
[0039] Hydroxyoxidation step:
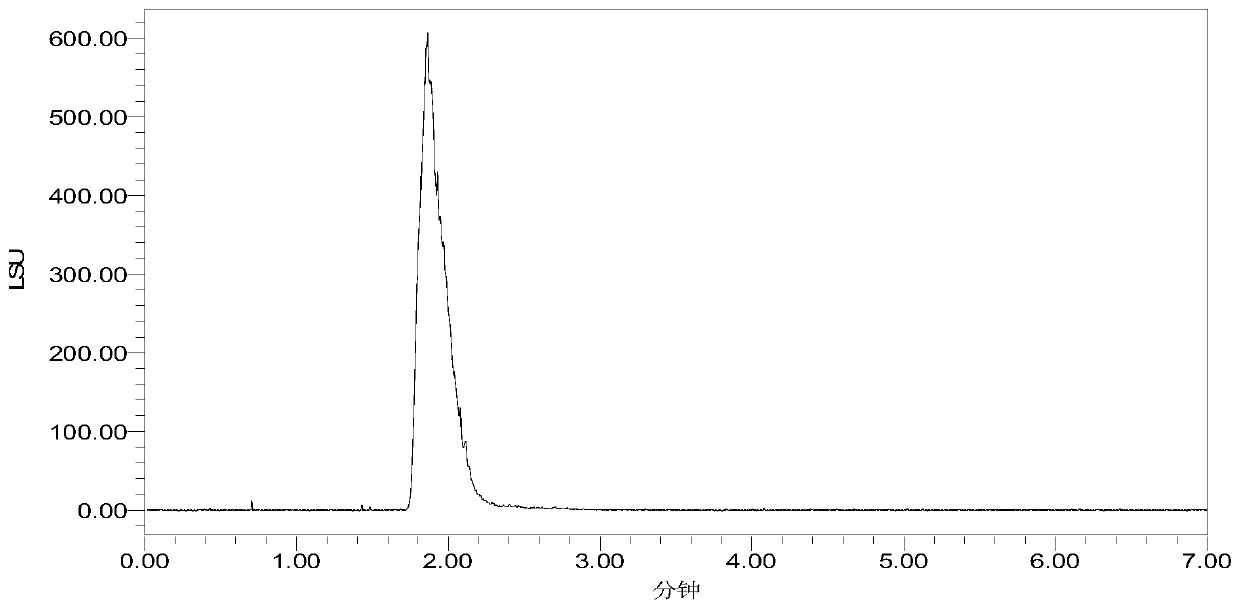
[0040] Put 5 g of the product obtained in Example 1 into a three-necked flask, dissolve it in 60 mL of toluene, cool it down to 0 ° C, add a mixed solution of 2 g of aluminum tert-butoxide and 10 mL of cyclohexanone and alkali-containing chloroform, then raise the temperature to 10 ° C, and stir After 4 hours of reaction, the end point of the reaction was monitored by thin-layer chromatography, and the reaction was completed. After the reaction, it was extracted with ethyl acetate, and the extract was concentrated under reduced pressure and dried in vacuo. 4.2 g of the oxidized product was obtained, the yield was 84%, and the melting point was 197-199°C.
[0041] The detection data of this product are as follows:
[0042] 1 HNMR(400MHz,DMSO):δ7.32-7.37(m,5H),5.09-5.25(m,2H),4.75(t,2H),4.51-4.57(m,3H),4.25(s,1H), 3.98-4.03(m,1H),3.57-3.60(m,2H),3.43-3.49(m,4H),3.33(s,3H),2.98-3.05(m,2H),2.52-2.68(m,3H ),2.31(d,J=11.3Hz,1H),2.26(s,8H),2.16(t,...
Embodiment 3
[0044] Carbonyl epoxidation steps:
[0045] Dissolve 5 g of the oxidation product obtained in Example 2 with a 50 / 100 mL (THF / DMSO) mixed solvent, weigh 3 g of NaH and 3 g of trimethylsulfonium iodide in DMSO, then add the oxidation product solution, and then add 2.5 mL triethylamine, stirred at 25°C for 3h. Thin-layer chromatography monitors the end point of the reaction, and the reaction ends. Add 50 mL of water, then add 100 mL of ethyl acetate, stir, separate the water phase, extract the ethyl acetate phase with water three times, dry over anhydrous sodium sulfate, concentrate under reduced pressure, and dry in vacuo to obtain 4 g of white solid with a yield of 79%, melting point: 189-191°C, elemental analysis: C 55.1, H 25.3, N 6.9.
[0046] The detection data of this product are as follows:
[0047] 1 HNMR (400MHz, DMSO): δ7.28-7.37(m, 5H), 5.10-5.16(m, 2H), 5.05(d, J=5.1Hz, 1H), 4.73(t, 2H), 4.65(d, J=19.2Hz, 1H), 4.50-4.53(m, 1H), 4.20(d, J=6.8Hz, 1H), 3.61(d, J=2...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com