Cabazitaxel weakly basic derivatives and preparations thereof
A technology of cabazitaxel and its derivatives, which is applied in the field of medicine, can solve the problems of limiting the clinical application of drugs, toxic and side effects, etc., and achieve the effect of increasing anti-tumor effect, high drug loading, and reducing adverse reactions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
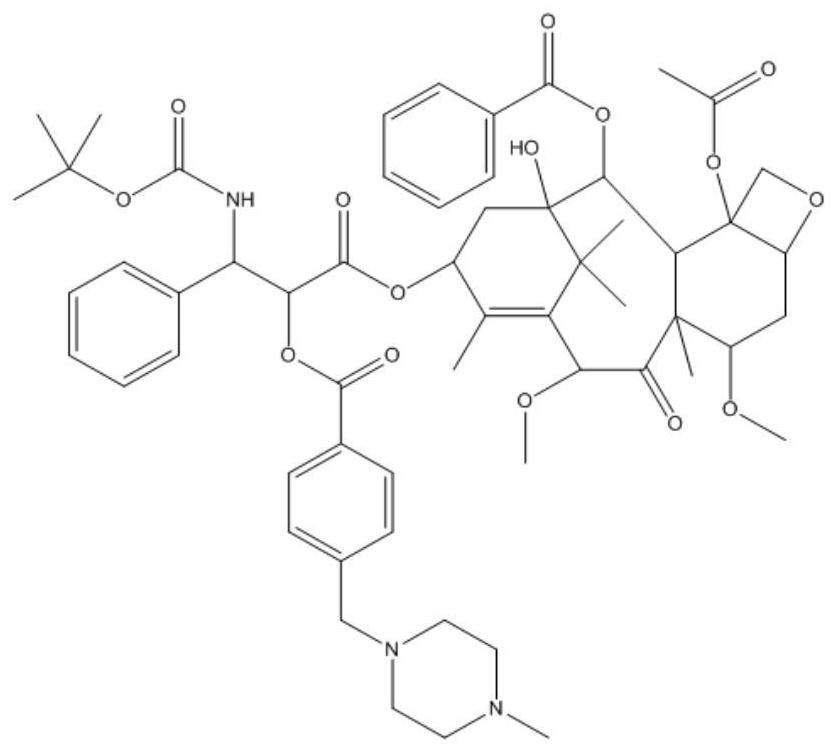
[0041] Example 1: Synthesis of a cabazitaxel derivative (CN1) whose base is 4-(4-methylpiperazinemethyl)phenyl
[0042] Weigh cabazitaxel (200mg, 0.24mmol) and 4-(4-methylpiperazinemethyl) benzoyl chloride (156mg, 0.48mmol) and dissolve in dichloromethane, add 0.25mL triethylamine, under ice-cooling Slowly add DMAP (5.9 mg, 0.048 mmol) in dichloromethane solution, N 2 Stir overnight at room temperature under protection. After the reaction was completed, it was separated and purified by column chromatography to obtain a white powder of the cabazitaxel derivative (yield 95.01%). Adopt proton nuclear magnetic resonance spectrum to determine the structure of compound in embodiment 1, the result is as follows figure 2 , the spectral analysis results are as follows:
[0043] 1H NMR (Chloroform-d, 400MHz) δ8.11 (2H, d, J = 7.4Hz), 7.94 (2H, d, J = 8.1 Hz), 7.61 (1H, t, J = 7.4Hz), 7.51 (1H ,d,J=7.8Hz),7.48(1H,d,J=7.8Hz),7.45– 7.35(6H,m),7.32–7.27(1H,m),6.26(1H,t,J=9.1Hz) ,5.64...
Embodiment 2
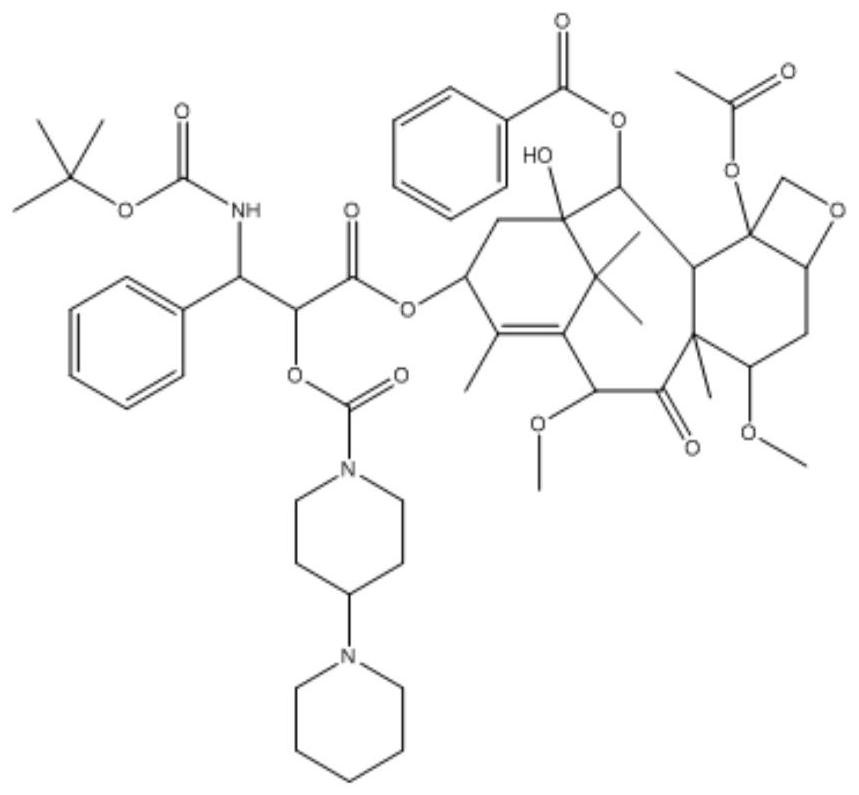
[0044] Example 2: Synthesis of Cabazitaxel derivatives (CN2) whose base is 4-(1-piperidinyl)piperidinyl
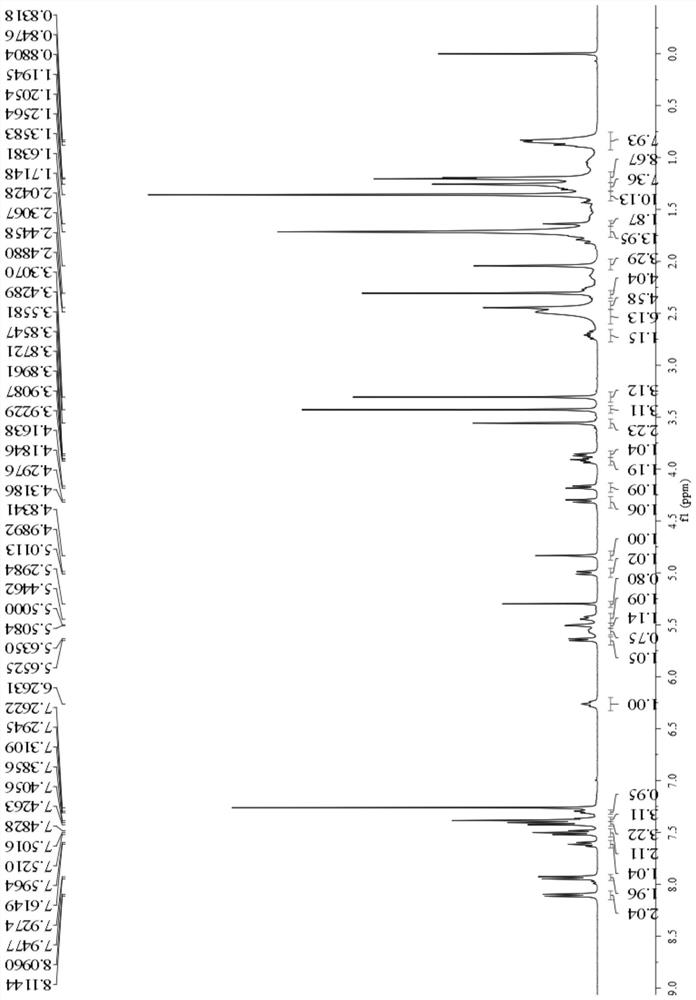
[0045] Weigh cabazitaxel (200mg, 0.24mmol) and 4-piperidinylpiperidine carboxylic acid chloride (111mg, 0.48mmol) and dissolve in dichloromethane, add 0.25mL triethylamine, slowly add DMAP (5.9 mg, 0.048mmol) in dichloromethane solution, N 2 Stir overnight at room temperature under protection. After the reaction was completed, it was separated and purified by column chromatography to obtain a white powder of the cabazitaxel derivative (yield 95%). Adopt proton nuclear magnetic resonance spectrum to determine the structure of compound in embodiment 2, the result is as follows Figure 4 , the spectral analysis results are as follows:
[0046] 1 H NMR (400MHz, Chloroform-d) δ8.07–8.00(m, 2H), 7.54(t, J=7.4Hz, 1H), 7.43(t, J=7.6Hz, 2H), 7.33(t, J= 7.6Hz, 2H), 7.22(m, 3H), 6.32–5.95(m, 1H), 5.56(d, J=7.0Hz, 1H), 5.44–5.26(m, 2H), 5.23(s, 1H), 5.20(d, J=3.8Hz, 1H), 4.92(d,...
Embodiment 3
[0047] Embodiment 3: the synthesis of the cabazitaxel derivative (CN3) that the base part is 4-methylpiperazine-1-methyl
[0048] Weigh cabazitaxel (200mg, 0.24mmol) and 4-methylpiperazine-1-formyl chloride (78mg, 0.48mmol) and dissolve in dichloromethane, add 0.25mL triethylamine, slowly add DMAP under ice-cooling (5.9mg, 0.048mmol) in dichloromethane, N 2 Stir overnight at room temperature under protection. After the reaction was completed, it was separated and purified by column chromatography to obtain a white powder of the cabazitaxel derivative (yield 93.8%). Adopt proton nuclear magnetic resonance spectrum to determine the structure of compound in embodiment 3, the result is as follows Image 6 , the spectral analysis results are as follows:
[0049] 1 H NMR (400MHz, Chloroform-d) δ8.03(d, J=7.5Hz, 2H), 7.54(t, J=7.4Hz, 1H), 7.43(t, J=7.6Hz, 2H), 7.32(t ,J=7.6Hz,2H),7.26–7.20(m,3H),6.17(t,J=9.3Hz,1H),5.56(d,J=7.0Hz,1H),5.36(s,1H),5.25 (s,1H),5.23(s,1H),4.92(dd, J=...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com